Taysha IPO Presentation Deck
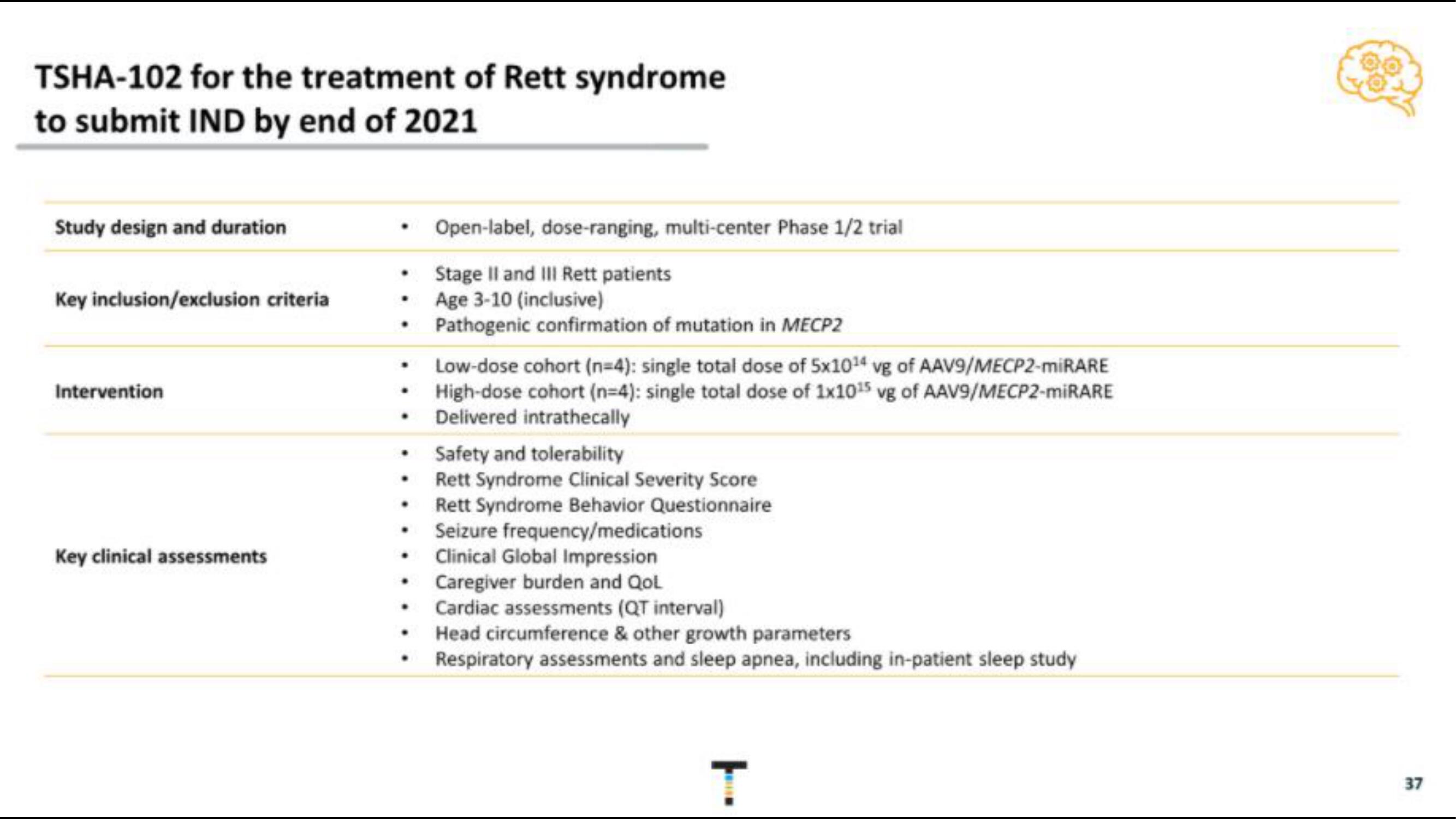
TSHA-102 for the treatment of Rett syndrome
to submit IND by end of 2021
Study design and duration
Key inclusion/exclusion criteria
Intervention
Key clinical assessments
Open-label, dose-ranging, multi-center Phase 1/2 trial
Stage II and III Rett patients
Age 3-10 (inclusive)
Pathogenic confirmation of mutation in MECP2
• Low-dose cohort (n=4): single total dose of 5x1014 vg of AAV9/MECP2-miRARE
High-dose cohort (n=4): single total dose of 1x1015 vg of AAV9/MECP2-miRARE
Delivered intrathecally
• Safety and tolerability
Rett Syndrome Clinical Severity Score
Rett Syndrome Behavior Questionnaire
Seizure frequency/medications
•
Clinical Global Impression
• Caregiver burden and Qol
.
Cardiac assessments (QT interval)
• Head circumference & other growth parameters
Respiratory assessments and sleep apnea, including in-patient sleep study
T
37View entire presentation