GreenLight SPAC Presentation Deck
CBH
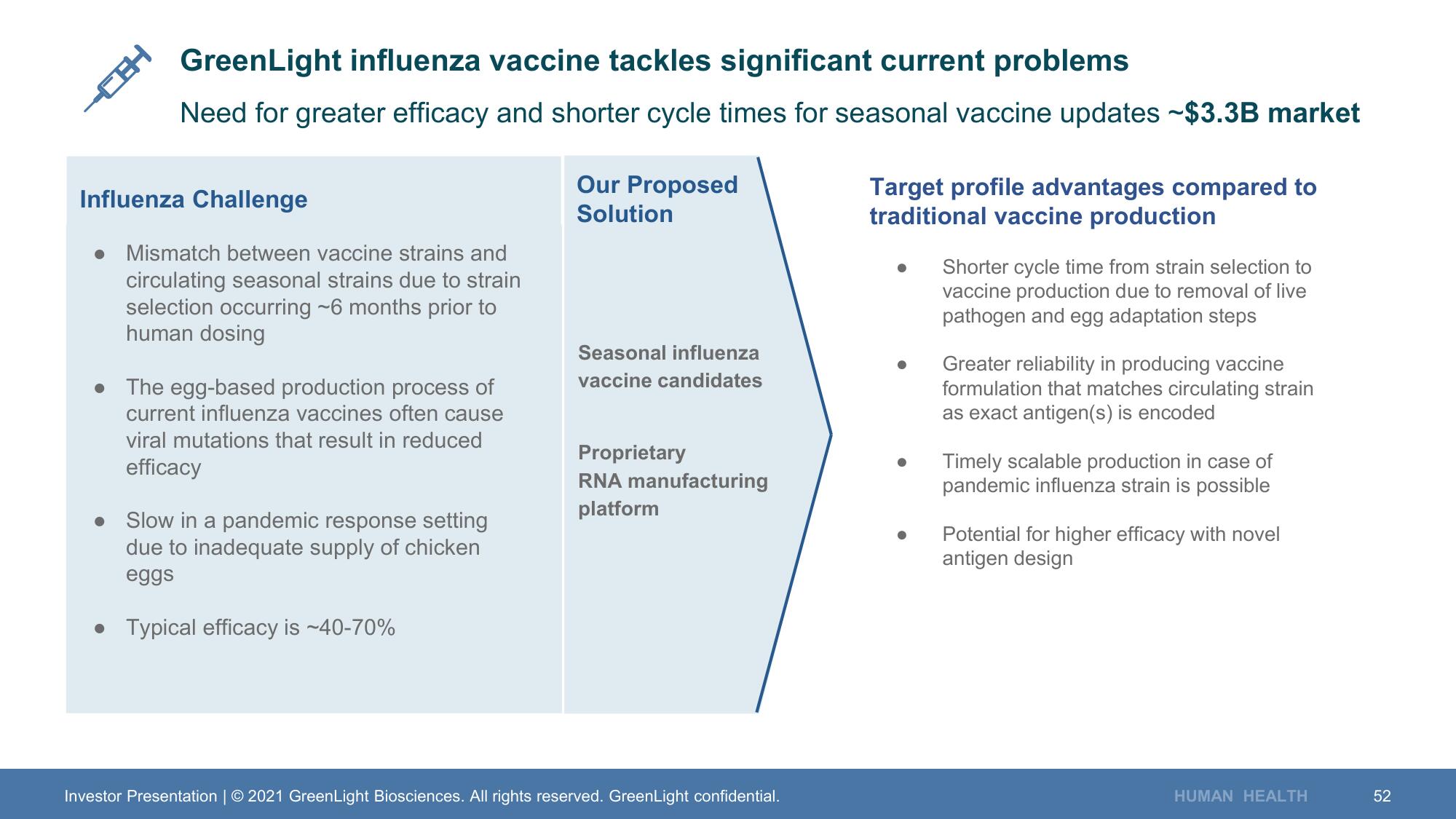
GreenLight influenza vaccine tackles significant current problems
Need for greater efficacy and shorter cycle times for seasonal vaccine updates ~$3.3B market
Influenza Challenge
Mismatch between vaccine strains and
circulating seasonal strains due to strain
selection occurring ~6 months prior to
human dosing
The egg-based production process of
current influenza vaccines often cause
viral mutations that result in reduced
efficacy
Slow in a pandemic response setting
due to inadequate supply of chicken
eggs
• Typical efficacy is ~40-70%
Our Proposed
Solution
Seasonal influenza
vaccine candidates
Proprietary
RNA manufacturing
platform
Investor Presentation | © 2021 GreenLight Biosciences. All rights reserved. GreenLight confidential.
Target profile advantages compared to
traditional vaccine production
●
Shorter cycle time from strain selection to
vaccine production due to removal of live
pathogen and egg adaptation steps
Greater reliability in producing vaccine
formulation that matches circulating strain
as exact antigen(s) is encoded
Timely scalable production in case of
pandemic influenza strain is possible
● Potential for higher efficacy with novel
antigen design
HUMAN HEALTH
52View entire presentation