Ocuphire Pharma Investor Day Presentation Deck
DR
DME
Phase 1/2 Clinical Trials: PK Data Supporting the ZETA-1 Trial
APX3330 Reaches the Retina via Oral Administration
Rat
Mouse
19
↑
Human
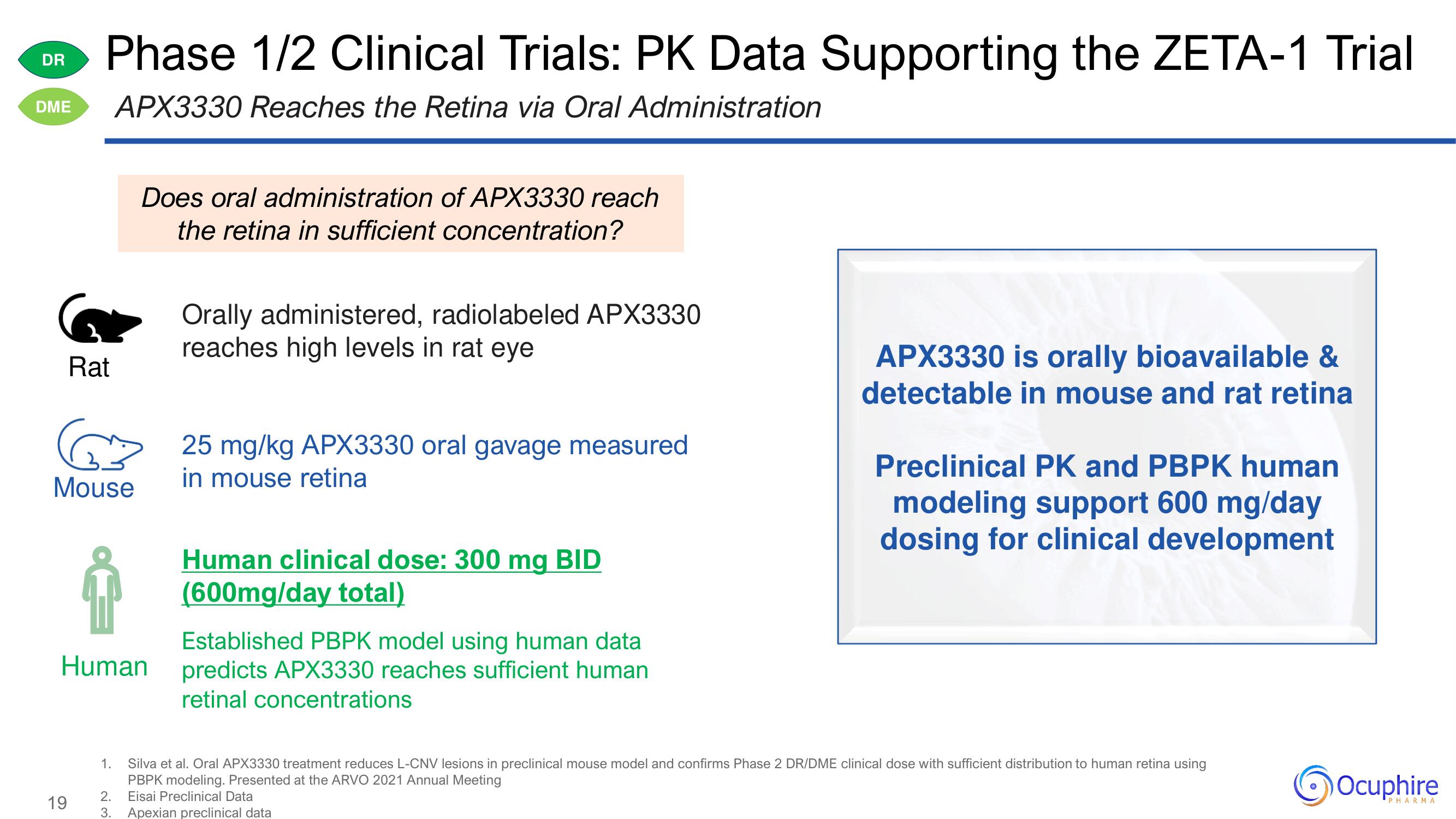
Does oral administration of APX3330 reach
the retina in sufficient concentration?
1.
Orally administered, radiolabeled APX3330
reaches high levels in rat eye
25 mg/kg APX3330 oral
25 mg/kg APX3330 oral gavage measured
in mouse retina
Human clinical dose: 300 mg BID
(600mg/day total)
Established PBPK model using human data
predicts APX3330 reaches sufficient human
retinal concentrations
APX3330 is orally bioavailable &
detectable in mouse and rat retina
2.
3. Apexian preclinical data
Preclinical PK and PBPK human
modeling support 600 mg/day
dosing for clinical development
Silva et al. Oral APX3330 treatment reduces L-CNV lesions in preclinical mouse model and confirms Phase 2 DR/DME clinical dose with sufficient distribution to human retina using
PBPK modeling. Presented at the ARVO 2021 Annual Meeting
Eisai Preclinical Data
Ocuphire
PHARMAView entire presentation