BioAtla IPO Presentation Deck
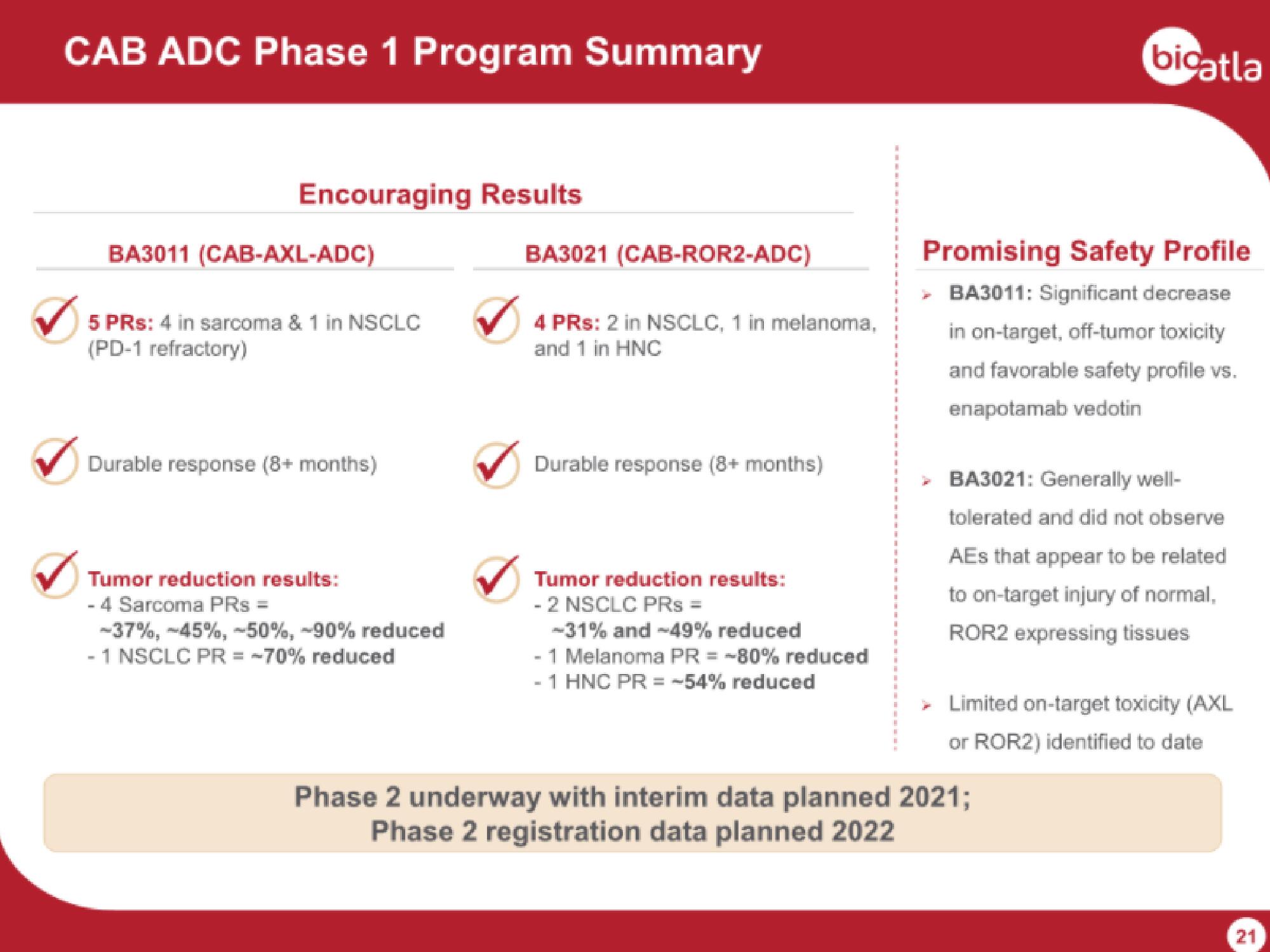
CAB ADC Phase 1 Program Summary
✔
✔
Encouraging Results
BA3011 (CAB-AXL-ADC)
5 PRs: 4 in sarcoma & 1 in NSCLC
(PD-1 refractory)
Durable response (8+ months)
Tumor reduction results:
- 4 Sarcoma PRs =
-37%, -45%, -50%, -90% reduced
- 1 NSCLC PR = -70% reduced
✔
✔
BA3021 (CAB-ROR2-ADC)
4 PRs: 2 in NSCLC, 1 in melanoma,
and 1 in HNC
Durable response (8+ months)
Tumor reduction results:
- 2 NSCLC PRs =
-31% and -49% reduced
F
- 1 Melanoma PR = -80% reduced
-1 HNC PR = -54% reduced
bicatla
Promising Safety Profile
> BA3011: Significant decrease
in on-target, off-tumor toxicity
and favorable safety profile vs.
enapotamab vedotin
> BA3021: Generally well-
tolerated and did not observe
AEs that appear to be related
to on-target injury of normal,
ROR2 expressing tissues
> Limited on-target toxicity (AXL
or ROR2) identified to date
Phase 2 underway with interim data planned 2021;
Phase 2 registration data planned 2022
21View entire presentation