Bionomics Investor Presentation Deck
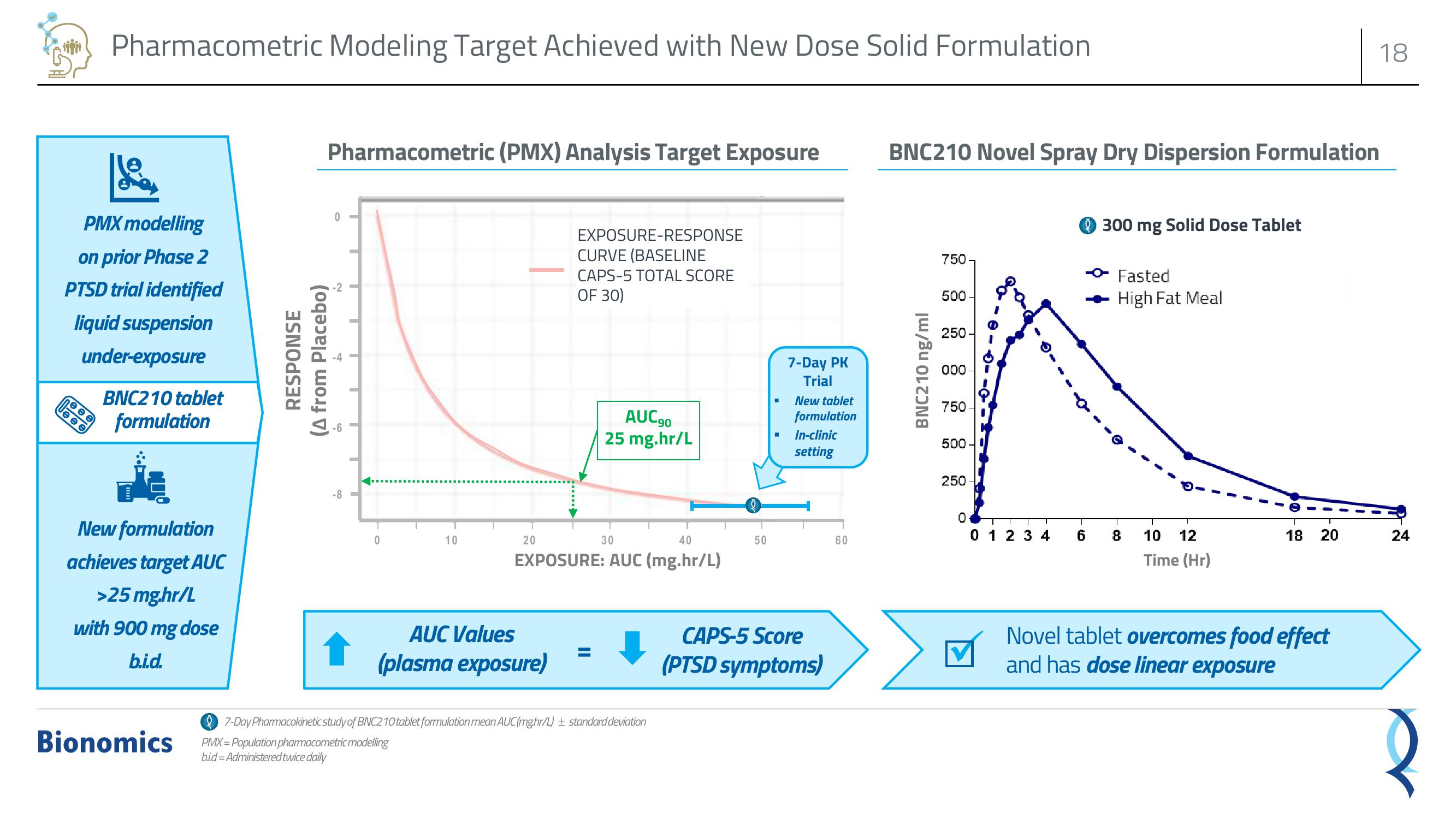
PMX modelling
on prior Phase 2
PTSD trial identified
liquid suspension
under-exposure
ne e
ee
O
Pharmacometric Modeling Target Achieved with New Dose Solid Formulation
e
BNC210 tablet
formulation
New formulation
achieves target AUC
>25 mg.hr/L
with 900 mg dose
b.i.d.
Bionomics
Pharmacometric (PMX) Analysis Target Exposure
RESPONSE
(A from Placebo)
0
-8
0
10
20
EXPOSURE-RESPONSE
CURVE (BASELINE
CAPS-5 TOTAL SCORE
OF 30)
30
40
EXPOSURE: AUC (mg.hr/L)
AUC Values
(plasma exposure)
AUC 90
25 mg.hr/L
=
7-Day Pharmacokinetic study of BNC2 10 tablet formulation mean AUC (mg.hr/L) ± standard deviation
PMX= Population pharmacometric modelling
b.id=Administered twice daily
50
7-Day PK
Trial
New tablet
formulation
In-clinic
setting
CAPS-5 Score
(PTSD symptoms)
60
BNC210 Novel Spray Dry Dispersion Formulation
BNC210 ng/ml
750
500-
250
000
750
500-
250
0
0 1 2 3 4
6
300 mg Solid Dose Tablet
-- Fasted
High Fat Meal
T
8
T
10 12
Time (Hr)
18 20
Novel tablet overcomes food effect
and has dose linear exposure
18
24View entire presentation