Valneva IPO Presentation Deck
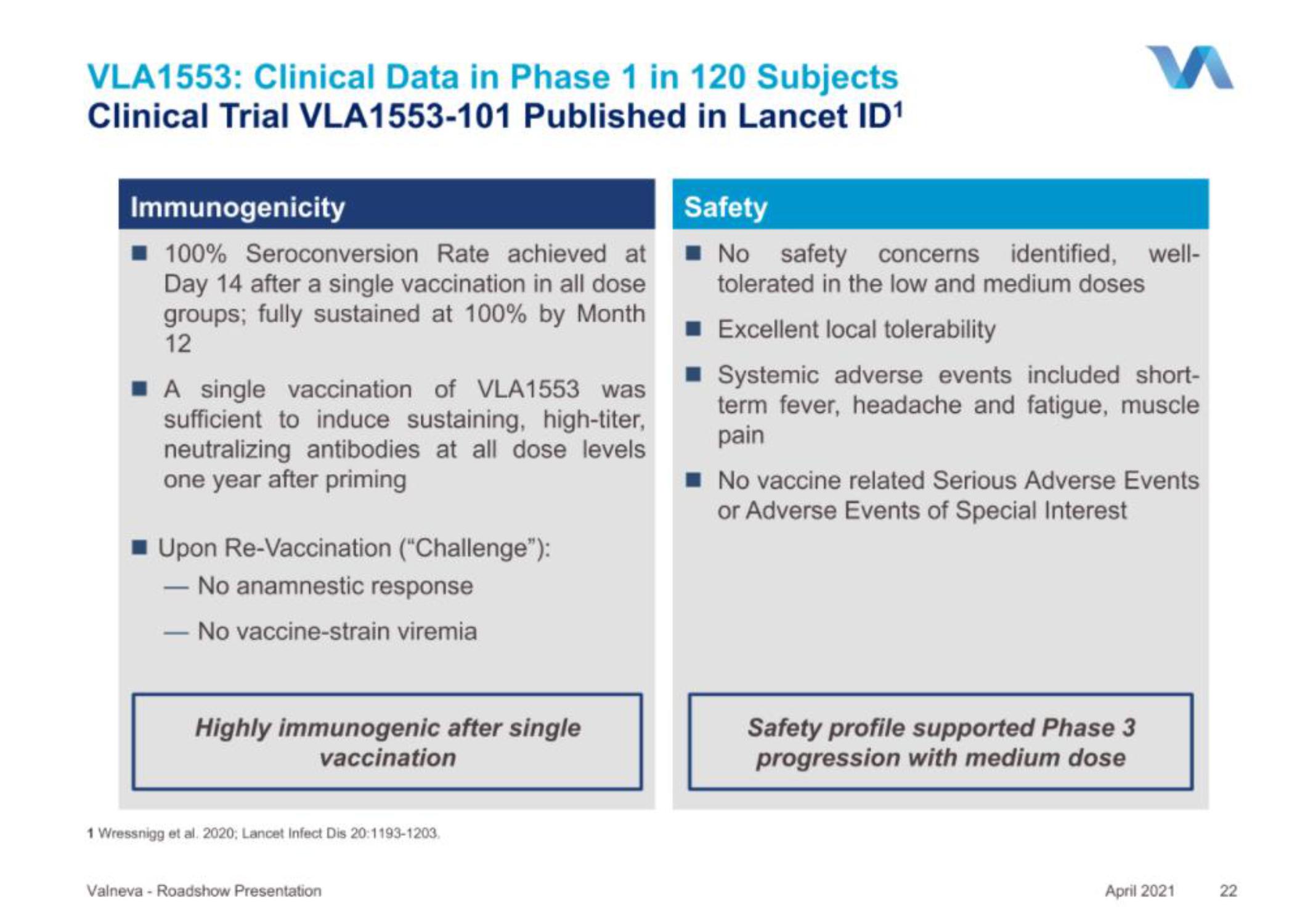
VLA1553: Clinical Data in Phase 1 in 120 Subjects
Clinical Trial VLA1553-101 Published in Lancet ID¹
Immunogenicity
100% Seroconversion Rate achieved at
Day 14 after a single vaccination in all dose
groups; fully sustained at 100% by Month
12
A single vaccination of VLA1553 was
sufficient to induce sustaining, high-titer,
neutralizing antibodies at all dose levels
one year after priming
■Upon Re-Vaccination ("Challenge"):
No anamnestic response
No vaccine-strain viremia
Highly immunogenic after single
vaccination
1 Wressnigg et al. 2020; Lancet Infect Dis 20:1193-1203.
Valneva - Roadshow Presentation
V
Safety
No safety concerns identified, well-
tolerated in the low and medium doses
Excellent local tolerability
■ Systemic adverse events included short-
term fever, headache and fatigue, muscle
pain
No vaccine related Serious Adverse Events
or Adverse Events of Special Interest
Safety profile supported Phase 3
progression with medium dose
April 2021
22View entire presentation