Certara Investor Presentation Deck
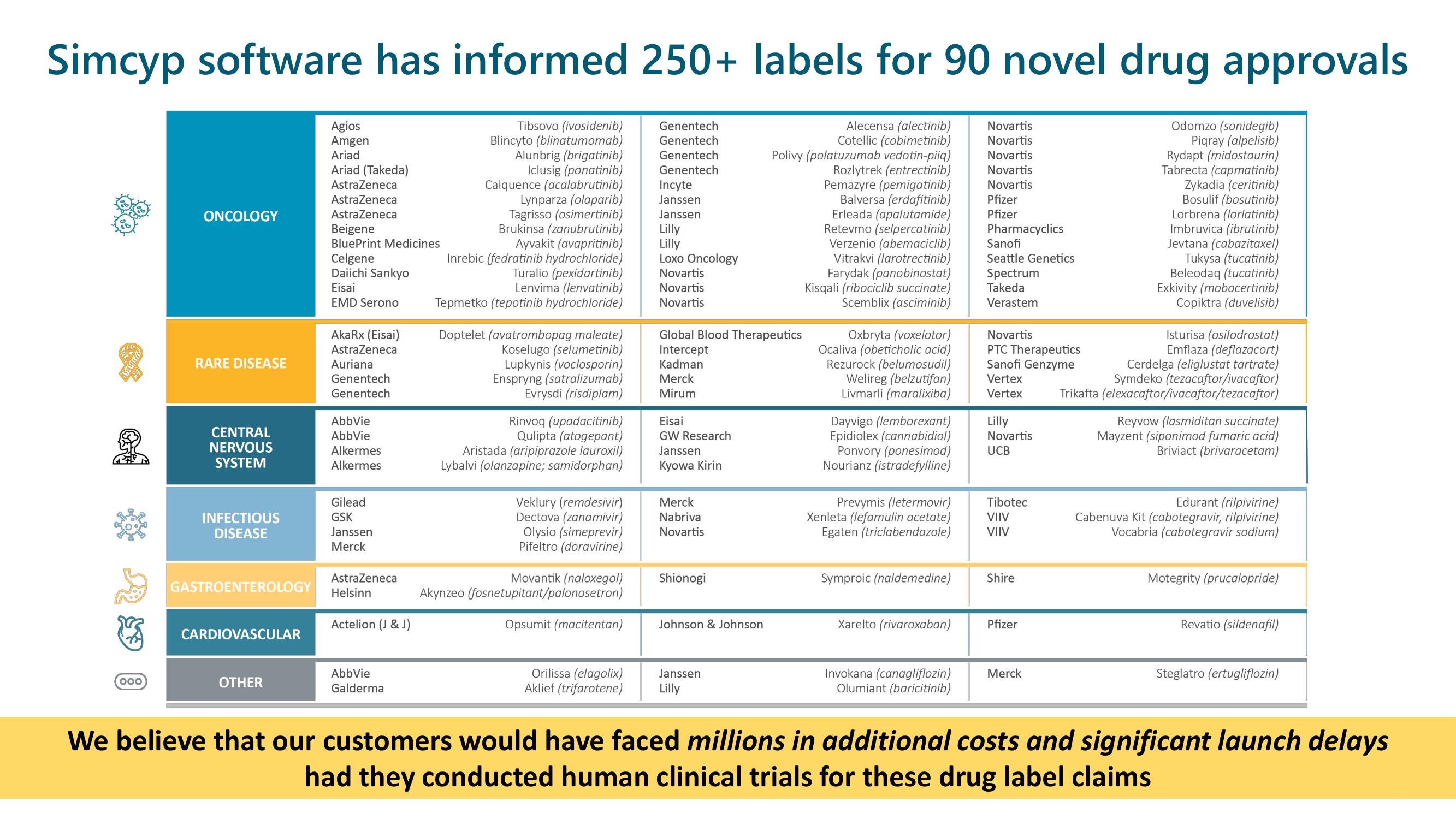
Simcyp software has informed 250+ labels for 90 novel drug approvals
Tibsovo (ivosidenib)
Blincyto (blinatumomab)
Alunbrig (brigatinib)
Iclusig (ponatinib)
Calquence (acalabrutinib)
Lynparza (olaparib)
Tagrisso (osimertinib)
Brukinsa (zanubrutinib)
Ayvakit (avapritinib)
Inrebic (fedratinib hydrochloride)
Turalio (pexidartinib)
Lenvima (lenvatinib)
Tepmetko (tepotinib hydrochloride)
Genentech
Genentech
Genentech
Genentech
Incyte
Janssen
Janssen
ASSO
ooo
ONCOLOGY
RARE DISEASE
CENTRAL
NERVOUS
SYSTEM
INFECTIOUS
DISEASE
GASTROENTEROLOGY
CARDIOVASCULAR
OTHER
Agios
Amgen
Ariad
Ariad (Takeda)
AstraZeneca
AstraZeneca
AstraZeneca
Beigene
BluePrint Medicines
Celgene
Daiichi Sankyo
Eisai
EMD Serono
AkaRx (Eisai)
AstraZeneca
Auriana
Genentech
Genentech
AbbVie
AbbVie
Alkermes
Alkermes
Gilead
GSK
Janssen
Merck
AstraZeneca
Helsinn
Actelion (J & J)
AbbVie
Galderma
Doptelet (avatrombopag maleate)
Koselugo (selumetinib)
Lupkynis (voclosporin)
Enspryng (satralizumab)
Evrysdi (risdiplam)
Rinvoq (upadacitinib)
Qulipta (atogepant)
Aristada (aripiprazole lauroxil)
Lybalvi (olanzapine; samidorphan)
Veklury (remdesivir)
Dectova (zanamivir)
Olysio (simeprevir)
Pifeltro (doravirine)
Movantik (naloxegol)
Akynzeo (fosnetupitant/palonosetron)
Opsumit (macitentan)
Orilissa (elagolix)
Aklief (trifarotene)
Lilly
Lilly
Loxo Oncology
Novartis
Novartis
Novartis
Global Blood Therapeutics
Intercept
Kadman
Merck
Mirum
Eisai
GW Research
Janssen
Kyowa Kirin
Merck
Nabriva
Novartis
Shionogi
Johnson & Johnson
Alecensa (alectinib) Novartis
Cotellic (cobimetinib) Novartis
Polivy (polatuzumab vedotin-piiq) Novartis
Rozlytrek (entrectinib) Novartis
Pemazyre (pemigatinib) Novartis
Balversa (erdafitinib) Pfizer
Erleada (apalutamide) Pfizer
Retevmo (selpercatinib) Pharmacyclics
Verzenio (abemaciclib) Sanofi
Vitrakvi (larotrectinib) Seattle Genetics
Farydak (panobinostat) Spectrum
Kisqali (ribociclib succinate) Takeda
Scemblix (asciminib) Verastem
Janssen
Lilly
Oxbryta (voxelotor)
Ocaliva (obeticholic acid)
Rezurock (belumosudil)
Welireg (belzutifan)
Livmarli (maralixiba)
Dayvigo (lemborexant)
Epidiolex (cannabidiol)
Ponvory (ponesimod)
Nourianz (istradefylline)
Prevymis (letermovir)
Xenleta (lefamulin acetate)
Egaten (triclabendazole)
Symproic (naldemedine)
Xarelto (rivaroxaban)
Invokana (canagliflozin)
Olumiant (baricitinib)
Novartis
PTC Therapeutics
Sanofi Genzyme
Vertex
Vertex
Lilly
Novartis
UCB
Tibotec
VIIV
VIIV
Shire
Pfizer
Merck
Odomzo (sonidegib)
Piqray (alpelisib)
Rydapt (midostaurin)
Tabrecta (capmatinib)
Zykadia (ceritinib)
Bosulif (bosutinib)
Lorbrena (lorlatinib)
Imbruvica (ibrutinib)
Jevtana (cabazitaxel)
Tukysa (tucatinib)
Beleodaq (tucatinib)
Exkivity (mobocertinib)
Copiktra (duvelisib)
Isturisa (osilodrostat)
Emflaza (deflazacort)
Cerdelga (eliglustat tartrate)
Symdeko (tezacaftor/ivacaftor)
Trikafta (elexacaftor/ivacaftor/tezacaftor)
Reyvow (lasmiditan succinate)
Mayzent (siponimod fumaric acid)
Briviact (brivaracetam)
Edurant (rilpivirine)
Cabenuva Kit (cabotegravir, rilpivirine)
Vocabria (cabotegravir sodium)
Motegrity (prucalopride)
Revatio (sildenafil)
Steglatro (ertugliflozin)
We believe that our customers would have faced millions in additional costs and significant launch delays
had they conducted human clinical trials for these drug label claimsView entire presentation