BioNTech Investor Day Presentation Deck
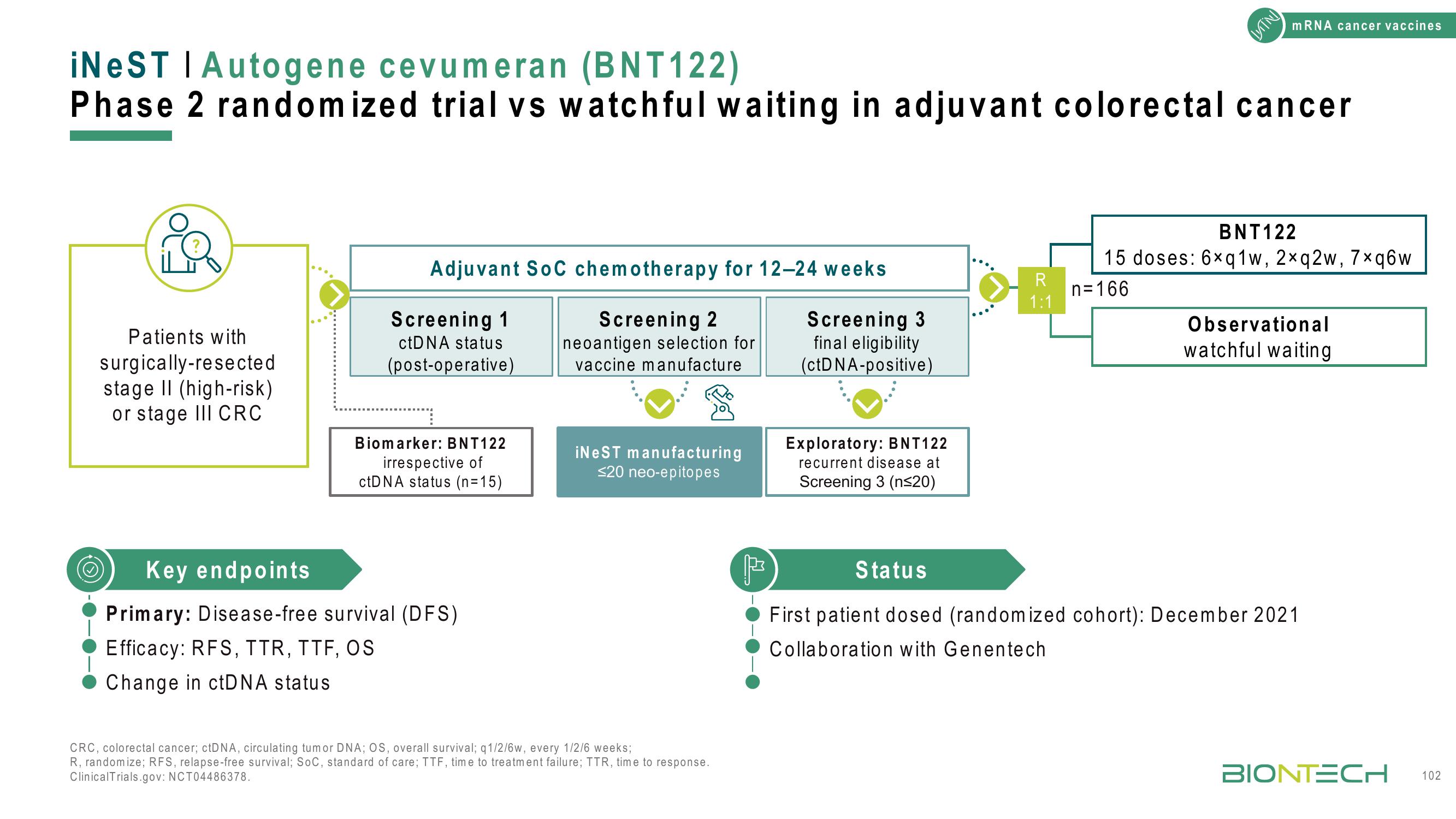
Patients with
surgically-resected.
stage II (high-risk)
or stage III CRC
iNeST | Autogene cevumeran (BNT122)
Phase 2 randomized trial vs watchful waiting in adjuvant colorectal cancer
Adjuvant SoC chemotherapy for 12-24 weeks
Screening 2
neoantigen selection for
vaccine manufacture
Screening 1
ctDNA status
(post-operative)
Biomarker: BNT122
irrespective of
ctDNA status (n=15)
Key endpoints
Primary: Disease-free survival (DFS)
Efficacy: RFS, TTR, TTF, OS
Change in ctDNA status.
iNeST manufacturing
≤20 neo-epitopes
CRC, colorectal cancer; ctDNA, circulating tumor DNA; OS, overall survival; q1/2/6w, every 1/2/6 weeks;
R, randomize; RFS, relapse-free survival; SoC, standard of care; TTF, time to treatment failure; TTR, time to response.
Clinical Trials.gov: NCT04486378.
S
M
Screening 3
final eligibility
(ctDNA-positive)
Exploratory: BNT122
recurrent disease at
Screening 3 (n≤20)
R
1:1
NUM
mRNA cancer vaccines
n=166
BNT122
15 doses: 6xq1w, 2xq2w, 7xq6w
Observational
watchful waiting
Status
First patient dosed (randomized cohort): December 2021
Collaboration with Genentech
BIONTECH
102View entire presentation