Ocuphire Pharma Investor Update
RM
15
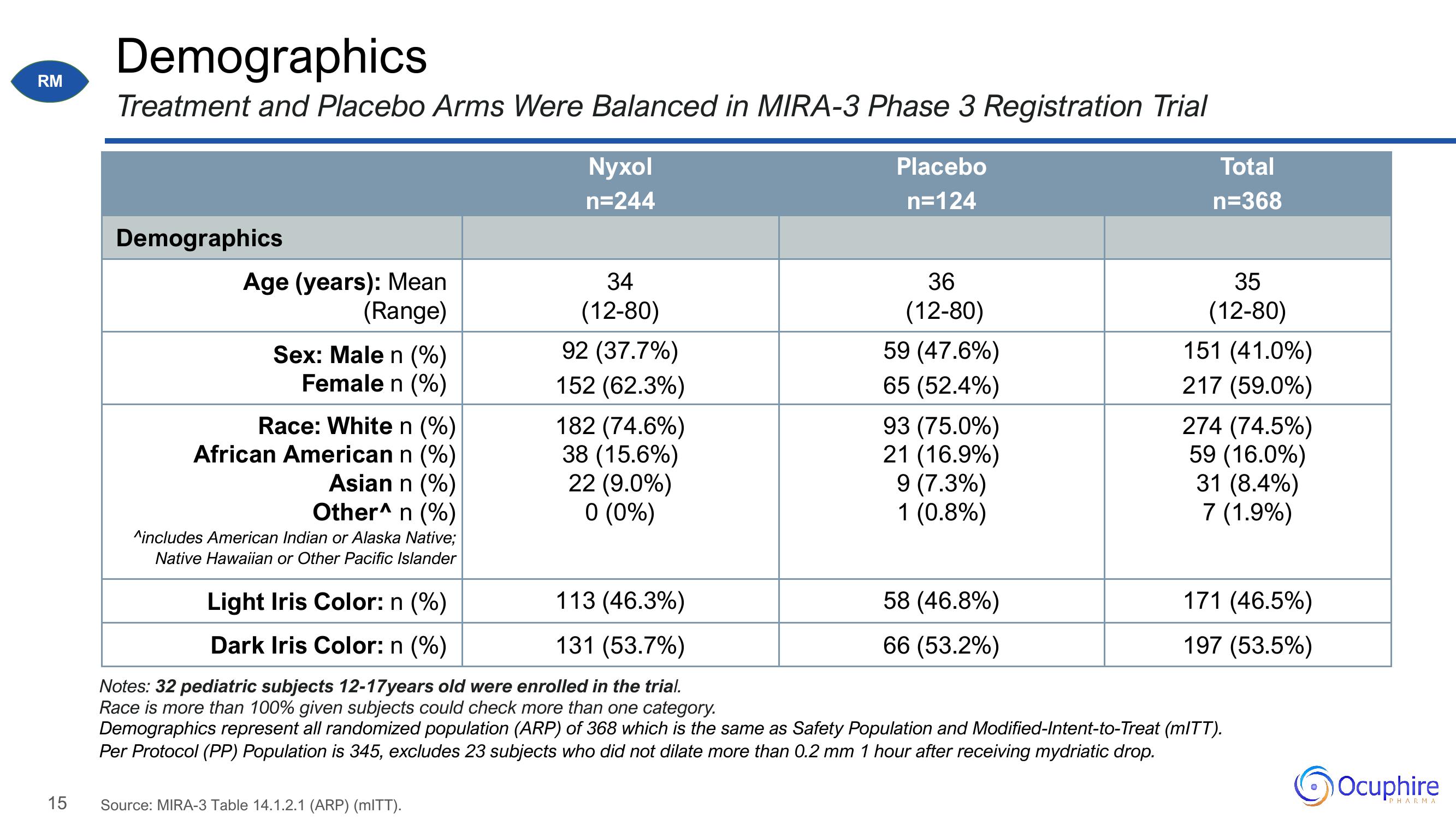
Demographics
Treatment and Placebo Arms Were Balanced in MIRA-3 Phase 3 Registration Trial
Demographics
Age (years): Mean
(Range)
Sex: Male n (%)
Female n (%)
Race: White n (%)
African American n (%)
Asian n (%)
Other^ n (%)
^includes American Indian or Alaska Native;
Native Hawaiian or Other Pacific Islander
Light Iris Color: n (%)
Dark Iris Color: n (%)
Nyxol
n=244
Source: MIRA-3 Table 14.1.2.1 (ARP) (MITT).
34
(12-80)
92 (37.7%)
152 (62.3%)
182 (74.6%)
38 (15.6%)
22 (9.0%)
0 (0%)
113 (46.3%)
131 (53.7%)
Placebo
n=124
36
(12-80)
59 (47.6%)
65 (52.4%)
93 (75.0%)
21 (16.9%)
9 (7.3%)
1 (0.8%)
58 (46.8%)
66 (53.2%)
Total
n=368
35
(12-80)
151 (41.0%)
217 (59.0%)
274 (74.5%)
59 (16.0%)
31 (8.4%)
7 (1.9%)
171 (46.5%)
197 (53.5%)
Notes: 32 pediatric subjects 12-17years old were enrolled in the trial.
Race is more than 100% given subjects could check more than one category.
Demographics represent all randomized population (ARP) of 368 which is the same as Safety Population and Modified-Intent-to-Treat (mITT).
Per Protocol (PP) Population is 345, excludes 23 subjects who did not dilate more than 0.2 mm 1 hour after receiving mydriatic drop.
Ocuphire
PHARMAView entire presentation