Nuvectis Pharma Investor Presentation Deck
Tumor Volume (mm³)
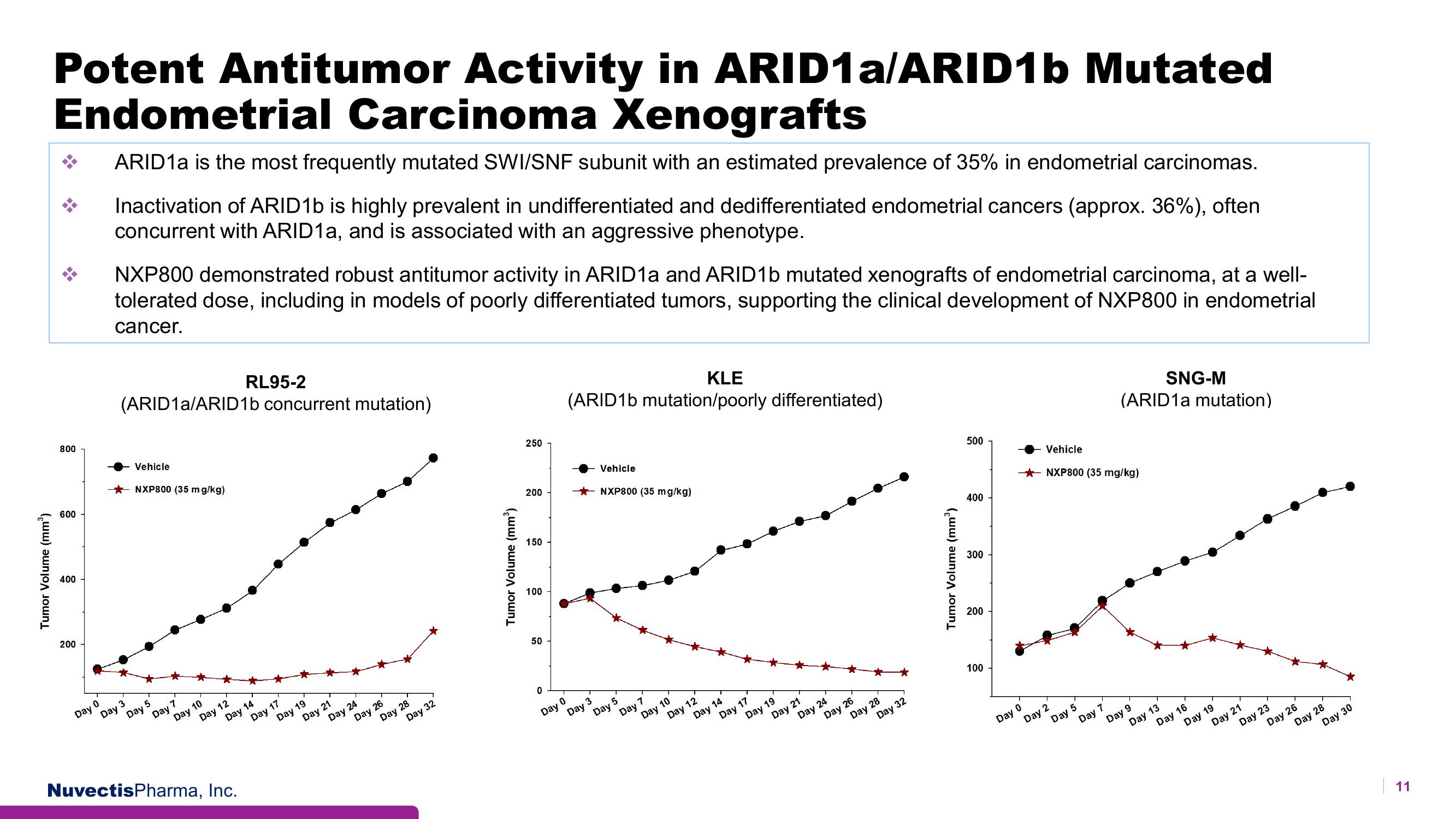
Potent Antitumor Activity in ARID1 a/ARID1b Mutated
Endometrial Carcinoma Xenografts
ARID1a is the most frequently mutated SWI/SNF subunit with an estimated prevalence of 35% in endometrial carcinomas.
Inactivation of ARID1b is highly prevalent in undifferentiated and dedifferentiated endometrial cancers (approx. 36%), often
concurrent with ARID1a, and is associated with an aggressive phenotype.
T
800
600
400
200
Day 0
NXP800 demonstrated robust antitumor activity in ARID1a and ARID1b mutated xenografts of endometrial carcinoma, at a well-
tolerated dose, including in models of poorly differentiated tumors, supporting the clinical development of NXP800 in endometrial
cancer.
RL95-2
(ARID1a/ARID1b concurrent mutation)
Vehicle
NXP800 (35 mg/kg)
Day 3
Day 5
Day 7
Day 12
Day 10
Day 14
NuvectisPharma, Inc.
Day 17
Day 19
Day 21
Day 24
Day 26
Day 28
Day 32
Tumor Volume (mm³)
250
200
150
100
50
0
Day 0
KLE
(ARID1b mutation/poorly differentiated)
Day 3
Vehicle
NXP800 (35 mg/kg)
Day 5
Day 7
Day 12
Day 10
Day 14
Day
Day 21
Day 19
Day 26
Day 24
Day 28
Day 32
Tumor Volume (mm³)
500
400
300
200
100
Vehicle
NXP800 (35 mg/kg)
Day 0
Day 2
Day 5
Day 7
(ARID1a mutation)
SNG-M
Day 9
Day 13
Day 16
Day 21
Day 19
Day 23
Day 26
Day 28
Day 30
11View entire presentation