Calliditas Therapeutics IPO Presentation Deck
Phase 3 trial design & criteria
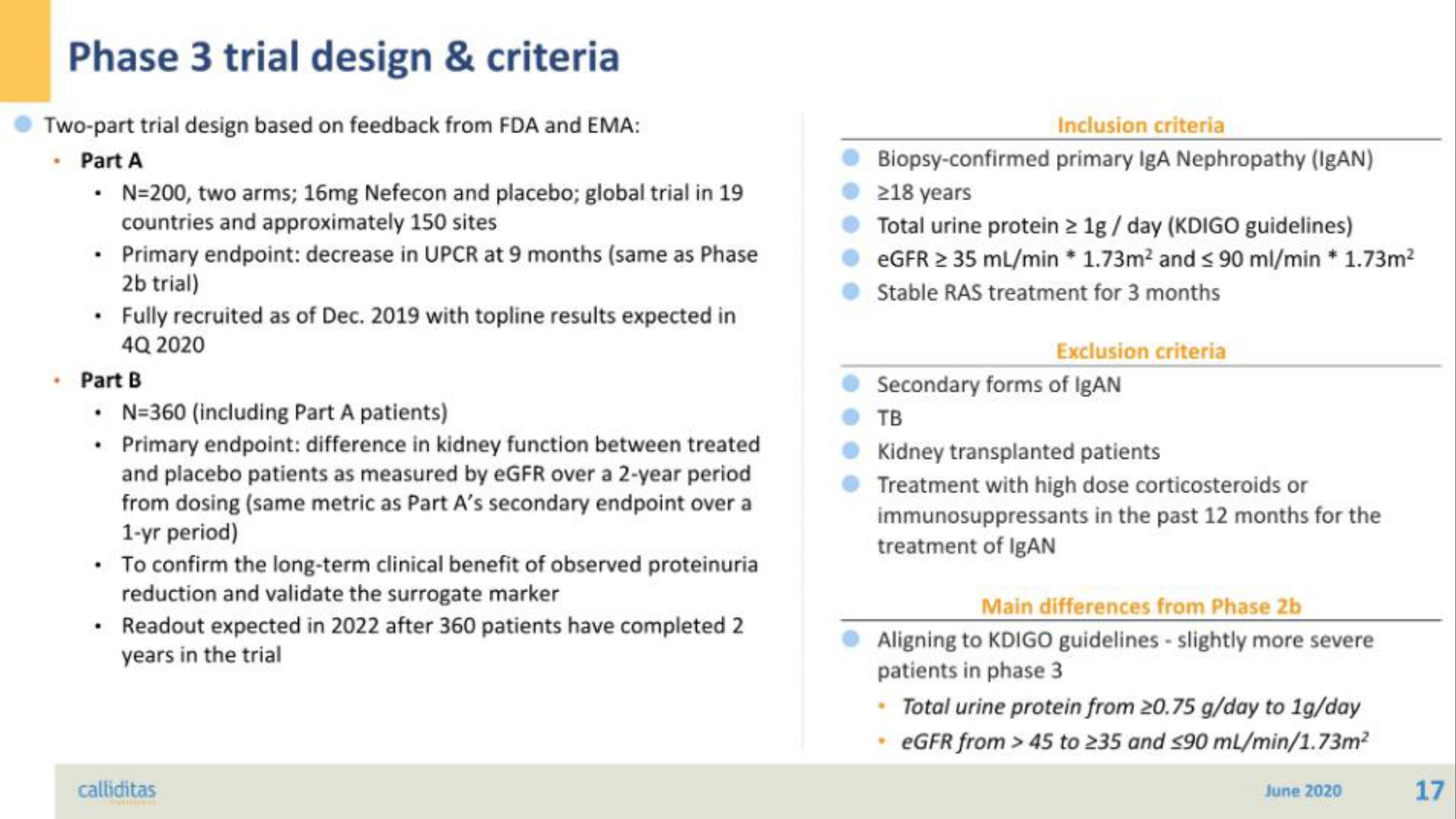
Two-part trial design based on feedback from FDA and EMA:
- Part A
• N=200, two arms; 16mg Nefecon and placebo; global trial in 19
countries and approximately 150 sites
• Primary endpoint: decrease in UPCR at 9 months (same as Phase
2b trial)
• Fully recruited as of Dec. 2019 with topline results expected in
4Q 2020
Part B
• N=360 (including Part A patients)
• Primary endpoint: difference in kidney function between treated
and placebo patients as measured by eGFR over a 2-year period.
from dosing (same metric as Part A's secondary endpoint over a
1-yr period)
.
To confirm the long-term clinical benefit of observed proteinuria
reduction and validate the surrogate marker
Readout expected in 2022 after 360 patients have completed 2
years in the trial
calliditas
Inclusion criteria
Biopsy-confirmed primary IgA Nephropathy (IgAN)
≥18 years
Total urine protein > 1g/day (KDIGO guidelines)
eGFR > 35 mL/min * 1.73m² and ≤ 90 ml/min * 1.73m²
Stable RAS treatment for 3 months
Exclusion criteria
Secondary forms of IgAN
TB
Kidney transplanted patients
Treatment with high dose corticosteroids or
immunosuppressants in the past 12 months for the
treatment of IgAN
Main differences from Phase 2b
Aligning to KDIGO guidelines - slightly more severe
patients in phase 3
• Total urine protein from 20.75 g/day to 1g/day
eGFR from > 45 to 235 and <90 mL/min/1.73m²
June 2020
17View entire presentation