Ocuphire Pharma Investor Update
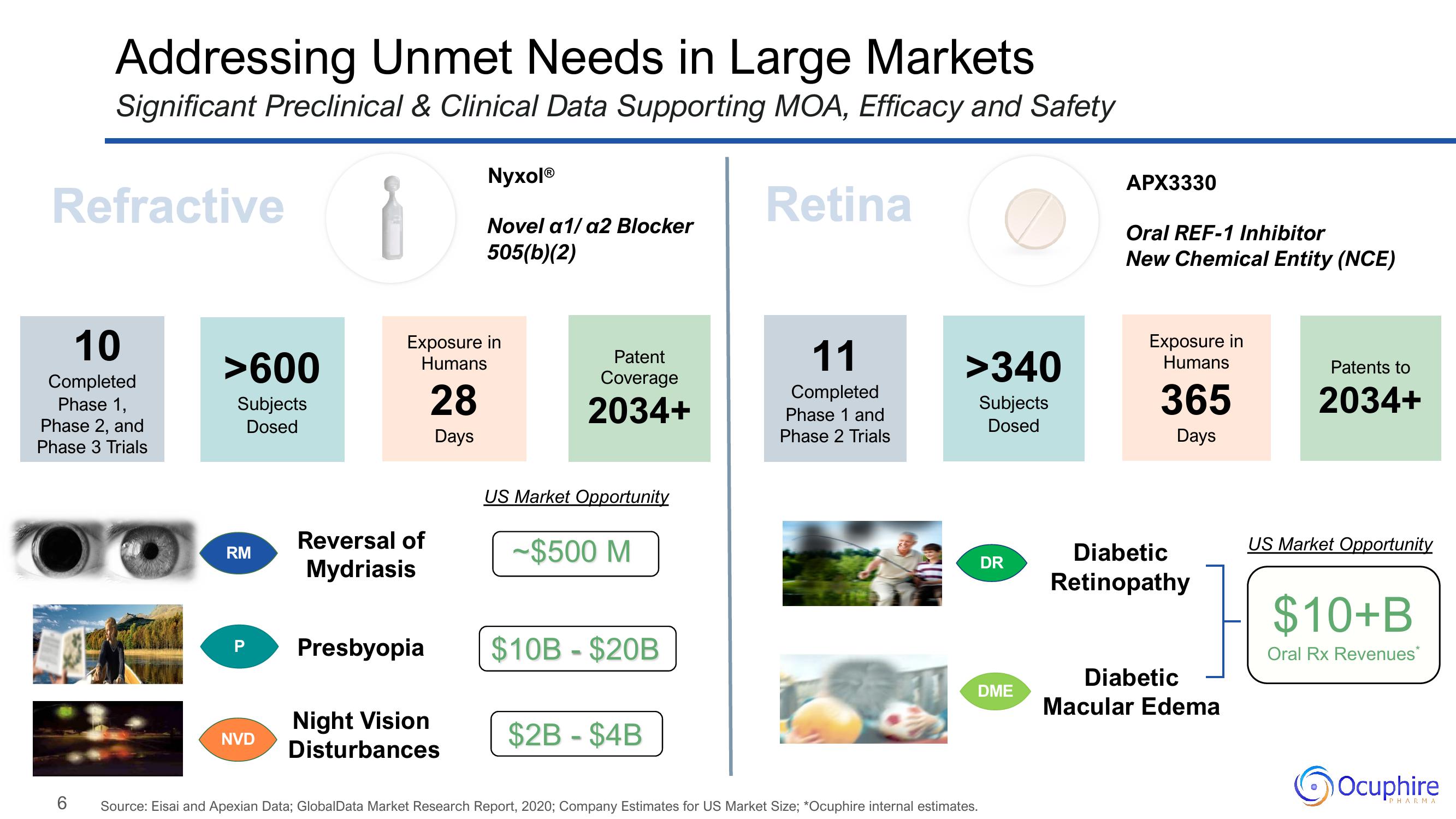
Addressing Unmet Needs in Large Markets
Significant Preclinical & Clinical Data Supporting MOA, Efficacy and Safety
Refractive
i
Retina
10
Completed
Phase 1,
Phase 2, and
Phase 3 Trials
CO
6
>600
Subjects
Dosed
RM
P
NVD
Exposure in
Humans
28
Days
Reversal of
Mydriasis
Presbyopia
NyxolⓇ
Novel a1/ a2 Blocker
505(b)(2)
Night Vision
Disturbances
Patent
Coverage
2034+
US Market Opportunity
-$500 M
$10B - $20B
$2B-$4B
11
Completed
Phase 1 and
Phase 2 Trials
>340
Subjects
Dosed
DR
DME
Source: Eisai and Apexian Data; GlobalData Market Research Report, 2020; Company Estimates for US Market Size; *Ocuphire internal estimates.
APX3330
Oral REF-1 Inhibitor
New Chemical Entity (NCE)
Exposure in
Humans
365
Days
Diabetic
Retinopathy
Diabetic
Macular Edema
Patents to
2034+
US Market Opportunity
$10+B
Oral Rx Revenues*
Ocuphire
PHARMAView entire presentation