BioAtla Investor Presentation Deck
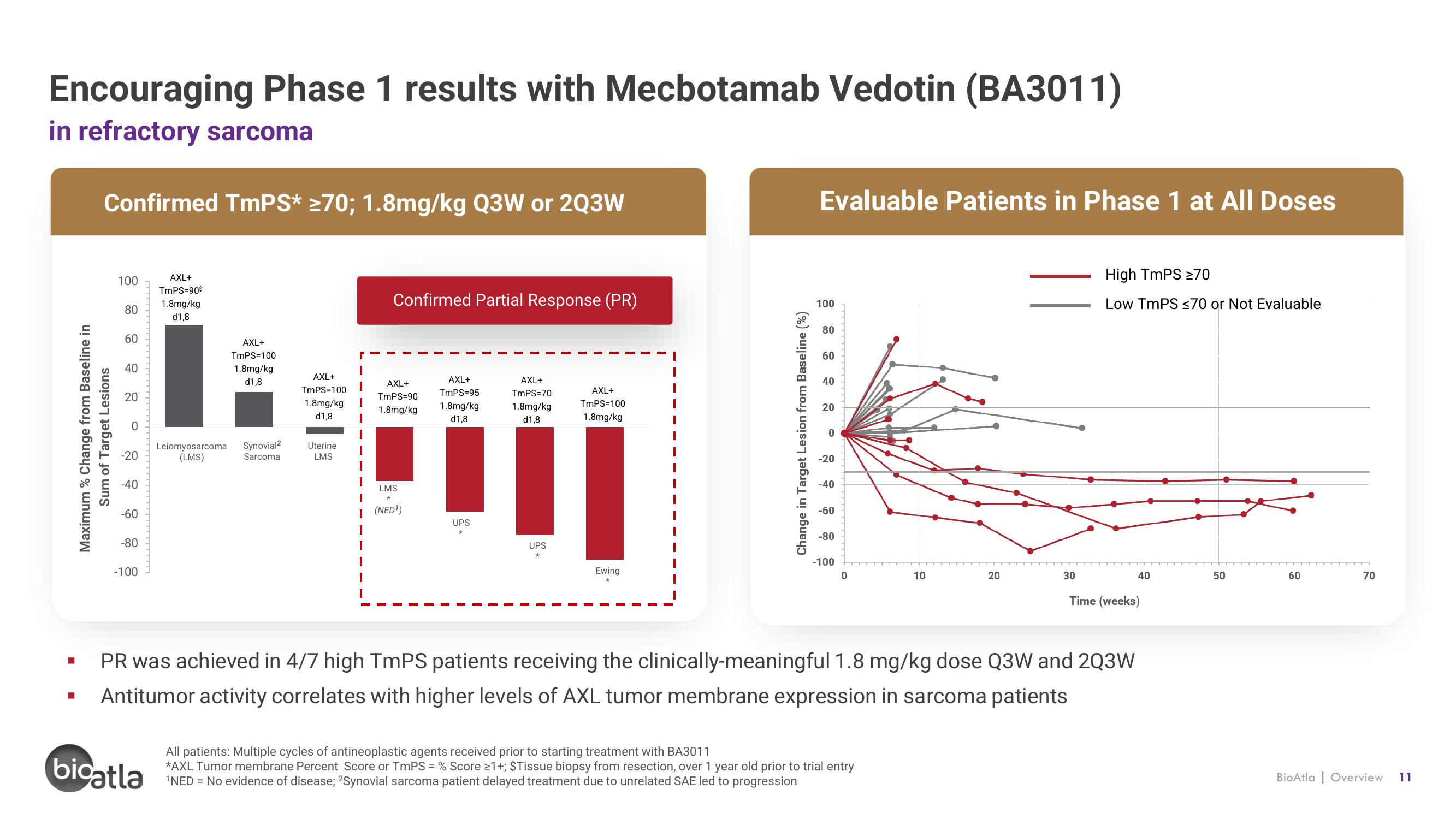
Encouraging Phase 1 results with Mecbotamab Vedotin (BA3011)
in refractory sarcoma
■
Confirmed TmPS* ≥70; 1.8mg/kg Q3W or 2Q3W
Maximum % Change from Baseline in
Sum of Target Lesions
100
80
60
40
20
-20
-40
-60
-80
-100
AXL+
TmPS-90$
bicatla
1.8mg/kg
d1,8
Leiomyosarcoma
(LMS)
AXL+
TmPS=100
1.8mg/kg
d1,8
Synovial²
Sarcoma
AXL+
TmPS=100
1.8mg/kg
d1,8
Uterine
LMS
r
Confirmed Partial Response (PR)
AXL+
TmPS-90
1.8mg/kg
LMS
*
(NED¹)
AXL+
TmPS-95
1.8mg/kg
d1,8
UPS
AXL+
TmPS-70
1.8mg/kg
d1,8
UPS
AXL+
TmPS=100
1.8mg/kg
Ewing
I
Change in Target Lesion from Baseline (%)
Evaluable Patients in Phase 1 at All Doses
100
80
60
40
20
-20
-40
-60
-80
-100
0
10
All patients: Multiple cycles of antineoplastic agents received prior to starting treatment with BA3011
*AXL Tumor membrane Percent Score or TmPS = % Score 21+; $Tissue biopsy from resection, over 1 year old prior to trial entry
¹NED = No evidence of disease; 2Synovial sarcoma patient delayed treatment due to unrelated SAE led to progression
20
=
30
High TmPS ≥70
Low TmPS ≤70 or Not Evaluable
PR was achieved in 4/7 high TmPS patients receiving the clinically-meaningful 1.8 mg/kg dose Q3W and 2Q3W
Antitumor activity correlates with higher levels of AXL tumor membrane expression in sarcoma patients
40
Time (weeks)
50
60
70
BioAtla| Overview
11View entire presentation