Aravive Investor Presentation Deck
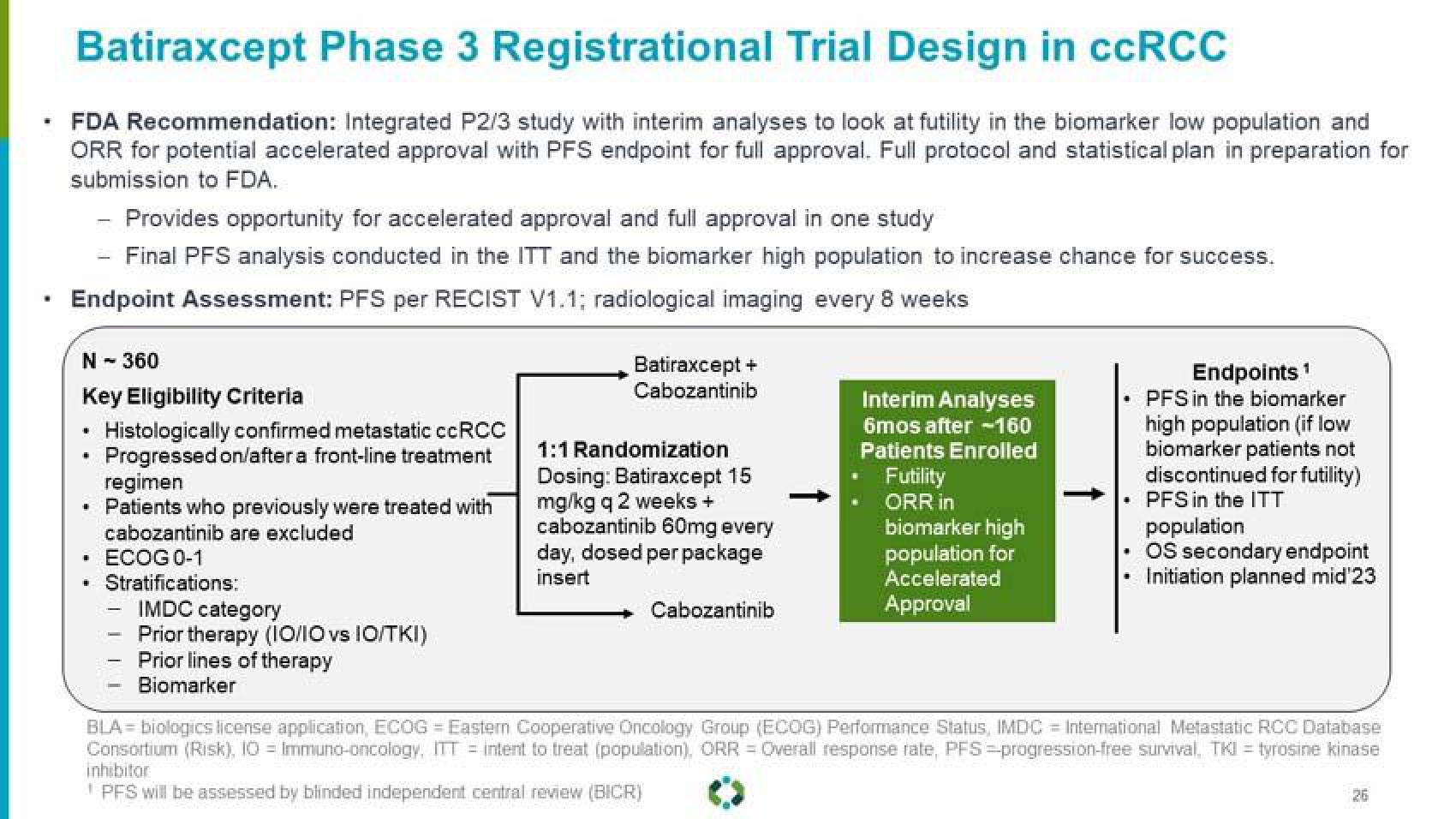
Batiraxcept Phase 3 Registrational Trial Design in ccRCC
FDA Recommendation: Integrated P2/3 study with interim analyses to look at futility in the biomarker low population and
ORR for potential accelerated approval with PFS endpoint for full approval. Full protocol and statistical plan in preparation for
submission to FDA.
Provides opportunity for accelerated approval and full approval in one study
Final PFS analysis conducted in the ITT and the biomarker high population to increase chance for success.
Endpoint Assessment: PFS per RECIST V1.1; radiological imaging every 8 weeks
N - 360
Key Eligibility Criteria
Histologically confirmed metastatic ccRCC
Progressed on/after a front-line treatment
regimen
• Patients who previously were treated with
cabozantinib are excluded
|
■
■
ECOG 0-1
Stratifications:
IMDC category
Prior therapy (10/10 vs 10/TKI)
Prior lines of therapy
Biomarker
Batiraxcept +
Cabozantinib
1:1 Randomization
Dosing: Batiraxcept 15
mg/kg q 2 weeks +
cabozantinib 60mg every
day, dosed per package
insert
Cabozantinib
Interim Analyses
6mos after -160
Patients Enrolled
Futility
ORR in
biomarker high
population for
Accelerated
Approval
i
1
Endpoints ¹
PFS in the biomarker
high population (if low
biomarker patients not
discontinued for futility)
• PFS in the ITT
population
• OS secondary endpoint
• Initiation planned mid'23
BLA= biologics license application, ECOG = Eastern Cooperative Oncology Group (ECOG) Performance Status, IMDC = International Metastatic RCC Database
onsortium (Risk), 10 = Immuno-oncology, ITT inte to treat (population), ORR = Overall response rate, PFS-progression-free survival, TKI = tyrosine kinase
inhibitor
¹PFS will be assessed by blinded independent central review (BICR)
26View entire presentation