Aravive Investor Presentation Deck
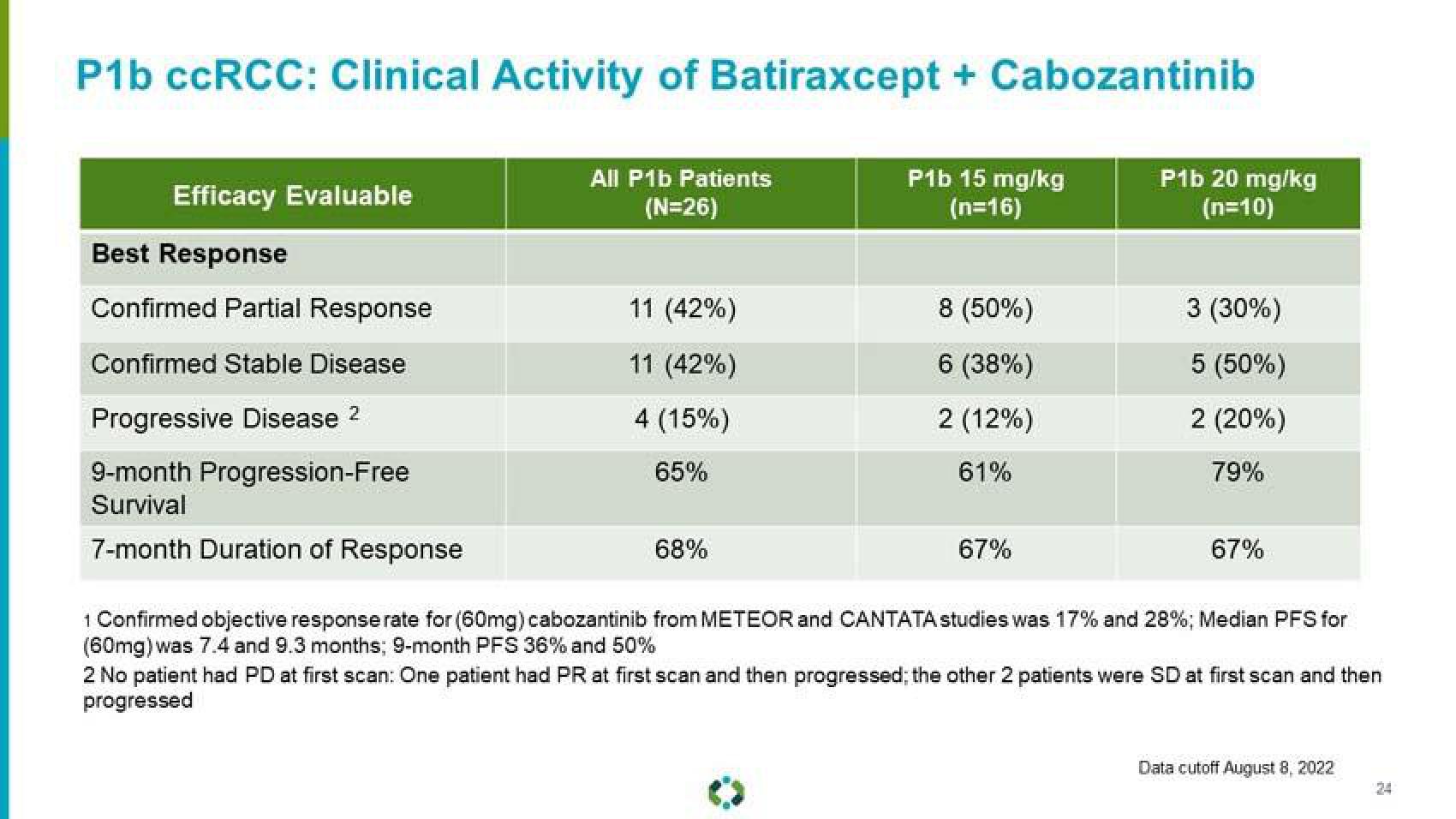
P1b ccRCC: Clinical Activity of Batiraxcept + Cabozantinib
All P1b Patients
(N=26)
P1b 15 mg/kg
(n=16)
Efficacy Evaluable
Best Response
Confirmed Partial Response
Confirmed Stable Disease
Progressive Disease 2
9-month Progression-Free
Survival
7-month Duration of Response
11 (42%)
11 (42%)
4 (15%)
65%
68%
8 (50%)
6 (38%)
2 (12%)
61%
67%
P1b 20 mg/kg
(n=10)
3 (30%)
5 (50%)
2 (20%)
79%
67%
1 Confirmed objective response rate for (60mg) cabozantinib from METEOR and CANTATA studies was 17% and 28%; Median PFS for
(60mg) was 7.4 and 9.3 months; 9-month PFS 36% and 50%
2 No patient had PD at first scan: One patient had PR at first scan and then progressed; the other 2 patients were SD at first scan and then
progressed
Data cutoff August 8, 2022
24View entire presentation