AstraZeneca Investor Day Presentation Deck
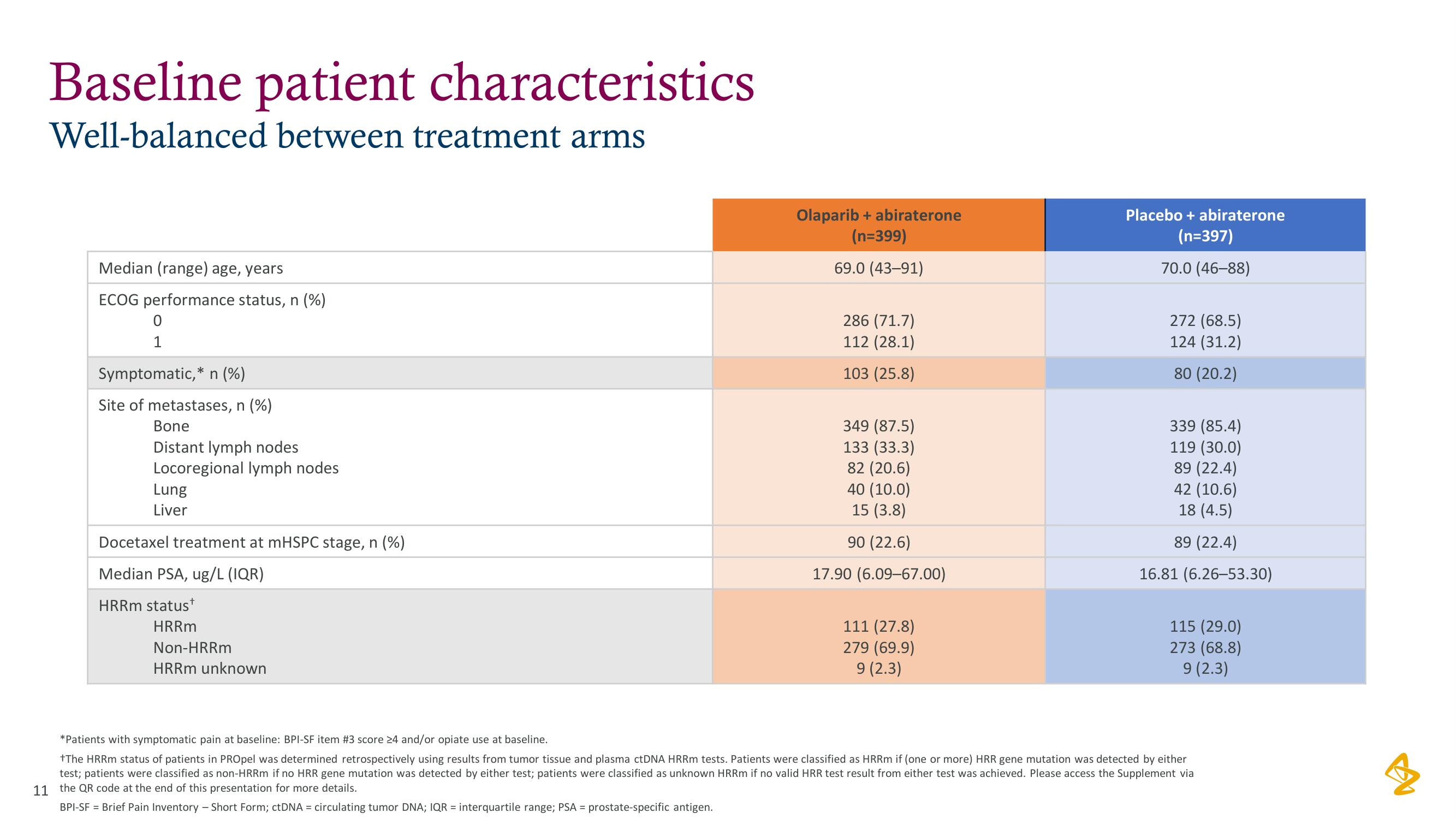
Baseline patient characteristics
Well-balanced between treatment arms
Median (range) age, years
ECOG performance status, n (%)
0
1
Symptomatic,* n (%)
Site of metastases, n (%)
Bone
Distant lymph nodes
Locoregional lymph nodes
Lung
Liver
Docetaxel treatment at mHSPC stage, n (%)
Median PSA, ug/L (IQR)
HRRm status*
HRRm
Non-HRRm
HRRm unknown
Olaparib + abiraterone
(n=399)
69.0 (43-91)
286 (71.7)
112 (28.1)
103 (25.8)
349 (87.5)
133 (33.3)
82 (20.6)
40 (10.0)
15 (3.8)
90 (22.6)
17.90 (6.09-67.00)
111 (27.8)
279 (69.9)
9 (2.3)
Placebo + abiraterone
(n=397)
70.0 (46-88)
272 (68.5)
124 (31.2)
80 (20.2)
339 (85.4)
119 (30.0)
89 (22.4)
42 (10.6)
18 (4.5)
89 (22.4)
16.81 (6.26-53.30)
115 (29.0)
273 (68.8)
9 (2.3)
*Patients with symptomatic pain at baseline: BPI-SF item #3 score 24 and/or opiate use at baseline.
+The HRRm status of patients in PROpel was determined retrospectively using results from tumor tissue and plasma ctDNA HRRm tests. Patients were classified as HRRm if (one or more) HRR gene mutation was detected by either
test; patients were classified as non-HRRm if no HRR gene mutation was detected by either test; patients were classified as unknown HRRm if no valid HRR test result from either test was achieved. Please access the Supplement via
11 the QR code at the end of this presentation for more details.
BPI-SF = Brief Pain Inventory - Short Form; ctDNA = circulating tumor DNA; IQR = interquartile range; PSA = prostate-specific antigen.
3View entire presentation