Ocuphire Pharma Investor Day Presentation Deck
RM
52
Percent of Subjects (%)
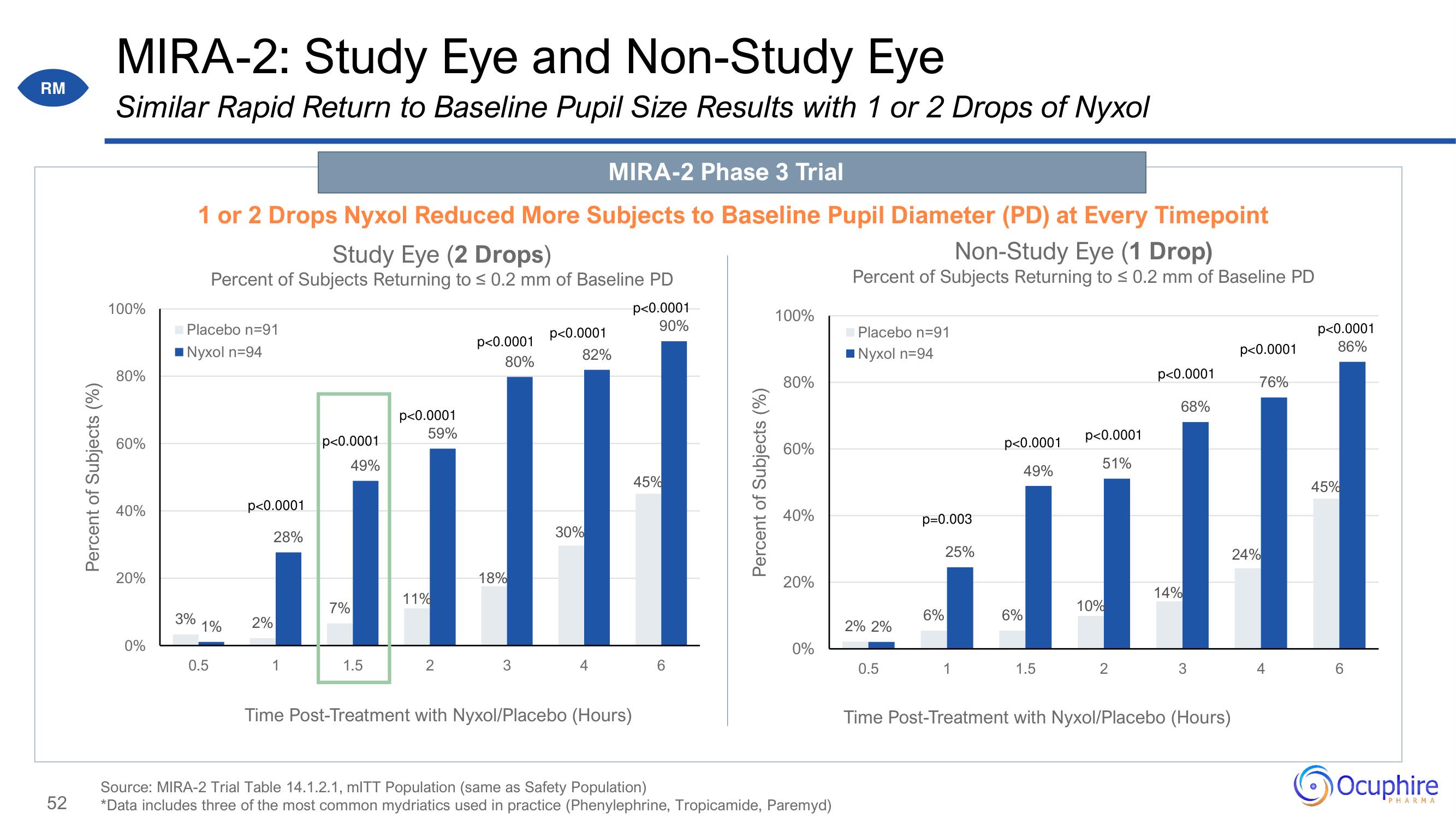
MIRA-2: Study Eye and Non-Study Eye
Similar Rapid Return to Baseline Pupil Size Results with 1 or 2 Drops of Nyxol
100%
80%
60%
40%
20%
0%
MIRA-2 Phase 3 Trial
1 or 2 Drops Nyxol Reduced More Subjects to Baseline Pupil Diameter (PD) at Every Timepoint
Non-Study Eye (1 Drop)
Study Eye (2 Drops)
Percent of Subjects Returning to ≤ 0.2 mm of Baseline PD
Percent of Subjects Returning to ≤ 0.2 mm of Baseline PD
Placebo n=91
Nyxol n=94
3%
1%
0.5
p<0.0001
2%
28%
1
p<0.0001
49%
7%
1.5
p<0.0001
59%
11%
2
p<0.0001
80%
18%
3
p<0.0001
82%
30%
4
Time Post-Treatment with Nyxol/Placebo (Hours)
p<0.0001
90%
45%
6
Percent of Subjects (%)
100%
80%
60%
40%
20%
0%
Source: MIRA-2 Trial Table 14.1.2.1, mITT Population (same as Safety Population)
*Data includes three of the most common mydriatics used in practice (Phenylephrine, Tropicamide, Paremyd)
Placebo n=91
Nyxol n=94
2% 2%
0.5
p=0.003
6%
25%
1
p<0.0001
49%
6%
1.5
p<0.0001
51%
10%
2
p<0.0001
68%
14%
3
Time Post-Treatment with Nyxol/Placebo (Hours)
p<0.0001
76%
24%
4
p<0.0001
86%
45%
6
Ocuphire
PHARMAView entire presentation