Ocuphire Pharma Results Presentation Deck
RM
7
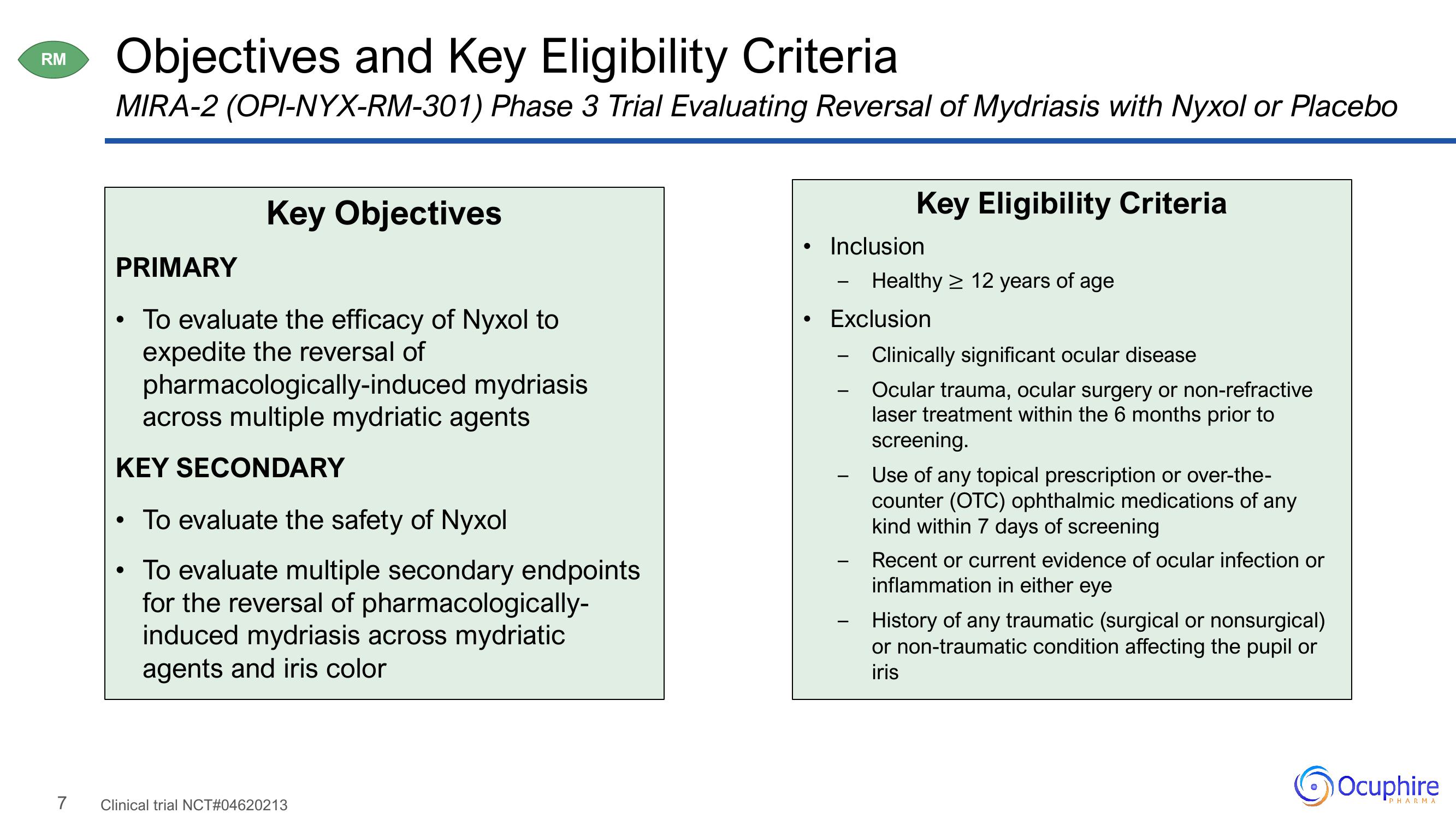
Objectives and Key Eligibility Criteria
MIRA-2 (OPI-NYX-RM-301) Phase 3 Trial Evaluating Reversal of Mydriasis with Nyxol or Placebo
Key Objectives
PRIMARY
To evaluate the efficacy of Nyxol to
expedite the reversal of
pharmacologically-induced
across multiple mydriatic agents
KEY SECONDARY
• To evaluate the safety of Nyxol
●
mydriasis
To evaluate multiple secondary endpoints
for the reversal of pharmacologically-
induced mydriasis across mydriatic
agents and iris color
Clinical trial NCT #04620213
●
Key Eligibility Criteria
Inclusion
Healthy ≥ 12 years of age
• Exclusion
-
Clinically significant ocular disease
Ocular trauma, ocular surgery or non-refractive
laser treatment within the 6 months prior to
screening.
- Use of any topical prescription or over-the-
counter (OTC) ophthalmic medications of any
kind within 7 days of screening
Recent or current evidence of ocular infection or
inflammation in either eye
History of any traumatic (surgical or nonsurgical)
or non-traumatic condition affecting the pupil or
iris
Ocuphire
PHARMAView entire presentation