Immix Biopharma Investor Presentation Deck
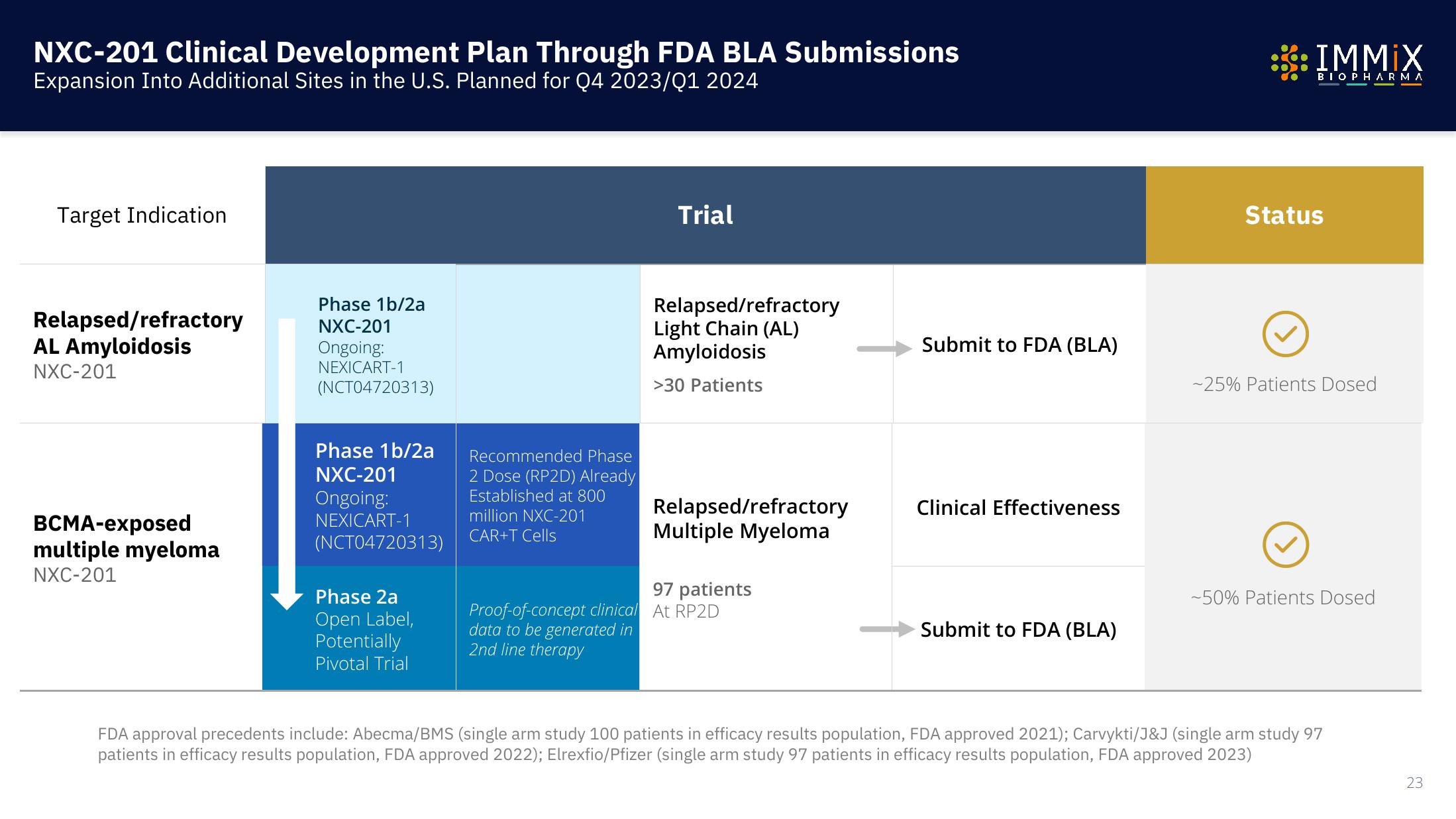
NXC-201 Clinical Development Plan Through FDA BLA Submissions
Expansion Into Additional Sites in the U.S. Planned for Q4 2023/01 2024
Target Indication
Relapsed/refractory
AL Amyloidosis
NXC-201
BCMA-exposed
multiple myeloma
NXC-201
Phase 1b/2a
NXC-201
Ongoing:
NEXICART-1
(NCT04720313)
Phase 1b/2a
NXC-201
Ongoing:
NEXICART-1
(NCT04720313)
Phase 2a
Open Label,
Potentially
Pivotal Trial
Recommended Phase
2 Dose (RP2D) Already
Established at 800
million NXC-201
CAR+T Cells
Trial
Relapsed/refractory
Light Chain (AL)
Amyloidosis
>30 Patients
Relapsed/refractory
Multiple Myeloma
97 patients
Proof-of-concept clinical At RP2D
data to be generated in
2nd line therapy
Submit to FDA (BLA)
Clinical Effectiveness
Submit to FDA (BLA)
●●●
IMMIX
S BIOPHARMA
Status
-25% Patients Dosed
-50% Patients Dosed
FDA approval precedents include: Abecma/BMS (single arm study 100 patients in efficacy results population, FDA approved 2021); Carvykti/J&J (single arm study 97
patients in efficacy results population, FDA approved 2022); Elrexfio/Pfizer (single arm study 97 patients in efficacy results population, FDA approved 2023)
23View entire presentation