AstraZeneca Results Presentation Deck
Respiratory & Immunology: anifrolumab
Potential first-in-class biologic medicine in lupus
Compelling data in resolution
of skin rash and arthritis
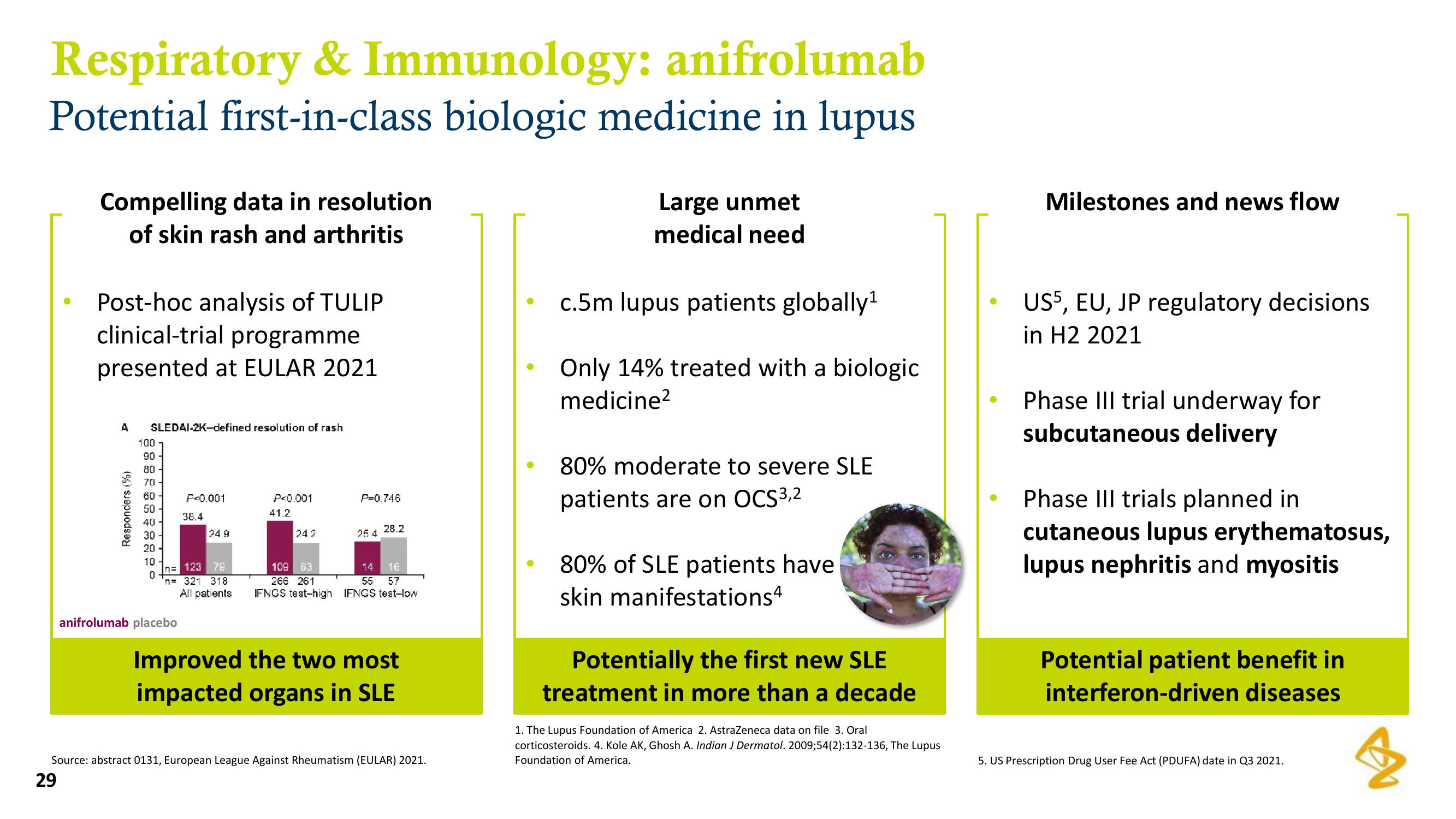
Post-hoc analysis of TULIP
clinical-trial programme
presented at EULAR 2021
A SLEDAI-2K-defined resolution of rash
Responders (%)
100
90
80
70
60
50
40
30
20
10
0
P<0.001
anifrolumab placebo
38.4
24.9
n= 123 79
'n= 321 318
All patients
P<0.001
41.2
24.2
P=0.746
25.4 28.2
109 63
14
16
266 261
57
55
IFNCS test-high IFNCS test-low
Improved the two most
impacted organs in SLE
Source: abstract 0131, European League Against Rheumatism (EULAR) 2021.
29
Large unmet
medical need
c.5m lupus patients globally¹
Only 14% treated with a biologic
medicine²
80% moderate to severe SLE
patients are on OCS3,2
80% of SLE patients have
skin manifestations4
Potentially the first new SLE
treatment in more than a decade
1. The Lupus Foundation of America 2. AstraZeneca data on file 3. Oral
corticosteroids. 4. Kole AK, Ghosh A. Indian J Dermatol. 2009;54(2):132-136, The Lupus
Foundation of America.
Milestones and news flow
US5, EU, JP regulatory decisions
in H2 2021
Phase III trial underway for
subcutaneous delivery
Phase III trials planned in
cutaneous lupus erythematosus,
lupus nephritis and myositis
Potential patient benefit in
interferon-driven diseases
5. US Prescription Drug User Fee Act (PDUFA) date in Q3 2021.
BView entire presentation