AstraZeneca Results Presentation Deck
Rare Disease - R&D highlights
Accelerating in ALXN2220 to Phase III for ATTR-CM
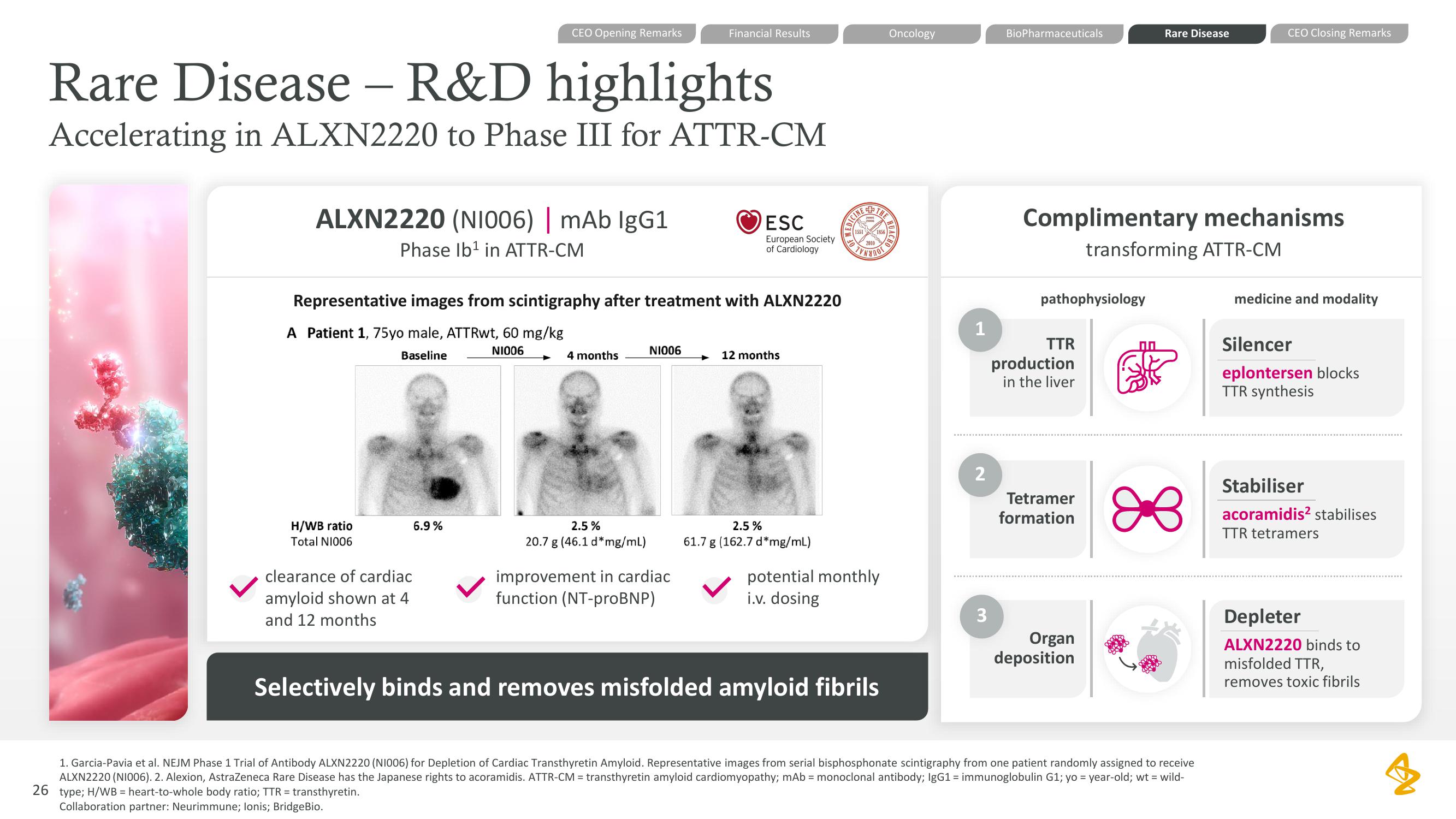
ALXN2220 (NI006) | mAb IgG1
Phase Ib¹ in ATTR-CM
CEO Opening Remarks
H/WB ratio
Total N1006
clearance of cardiac
amyloid shown at 4
and 12 months
Representative images from scintigraphy after treatment with ALXN2220
A Patient 1, 75yo male, ATTRwt, 60 mg/kg
N1006
Baseline
6.9%
4 months
2.5%
20.7 g (46.1 d* mg/ml)
Financial Results
NI006
improvement in cardiac
function (NT-proBNP)
ESC
European Society
of Cardiology
+ 12 months
2.5%
61.7 g (162.7 d*mg/mL)
EDICINE
1551
2010
1856
potential monthly
i.v. dosing
Selectively binds and removes misfolded amyloid fibrils
Oncology
1
2
3
BioPharmaceuticals
pathophysiology
Complimentary mechanisms
transforming ATTR-CM
TTR
production
in the liver
Tetramer
formation
Organ
deposition
Rare Disease
ПО
∞
CEO Closing Remarks
1. Garcia-Pavia et al. NEJM Phase 1 Trial of Antibody ALXN2220 (N1006) for Depletion of Cardiac Transthyretin Amyloid. Representative images from serial bisphosphonate scintigraphy from one patient randomly assigned to receive
ALXN2220 (N1006). 2. Alexion, AstraZeneca Rare Disease has the Japanese rights to acoramidis. ATTR-CM = transthyretin amyloid cardiomyopathy; mAb = monoclonal antibody; IgG1 = immunoglobulin G1; yo = year-old; wt = wild-
26 type; H/WB = heart-to-whole body ratio; TTR = transthyretin.
Collaboration partner: Neurimmune; lonis; Bridge Bio.
medicine and modality
Silencer
eplontersen blocks
TTR synthesis
Stabiliser
acoramidis² stabilises
TTR tetramers
Depleter
ALXN2220 binds to
misfolded TTR,
removes toxic fibrilsView entire presentation