Equillium Results Presentation Deck
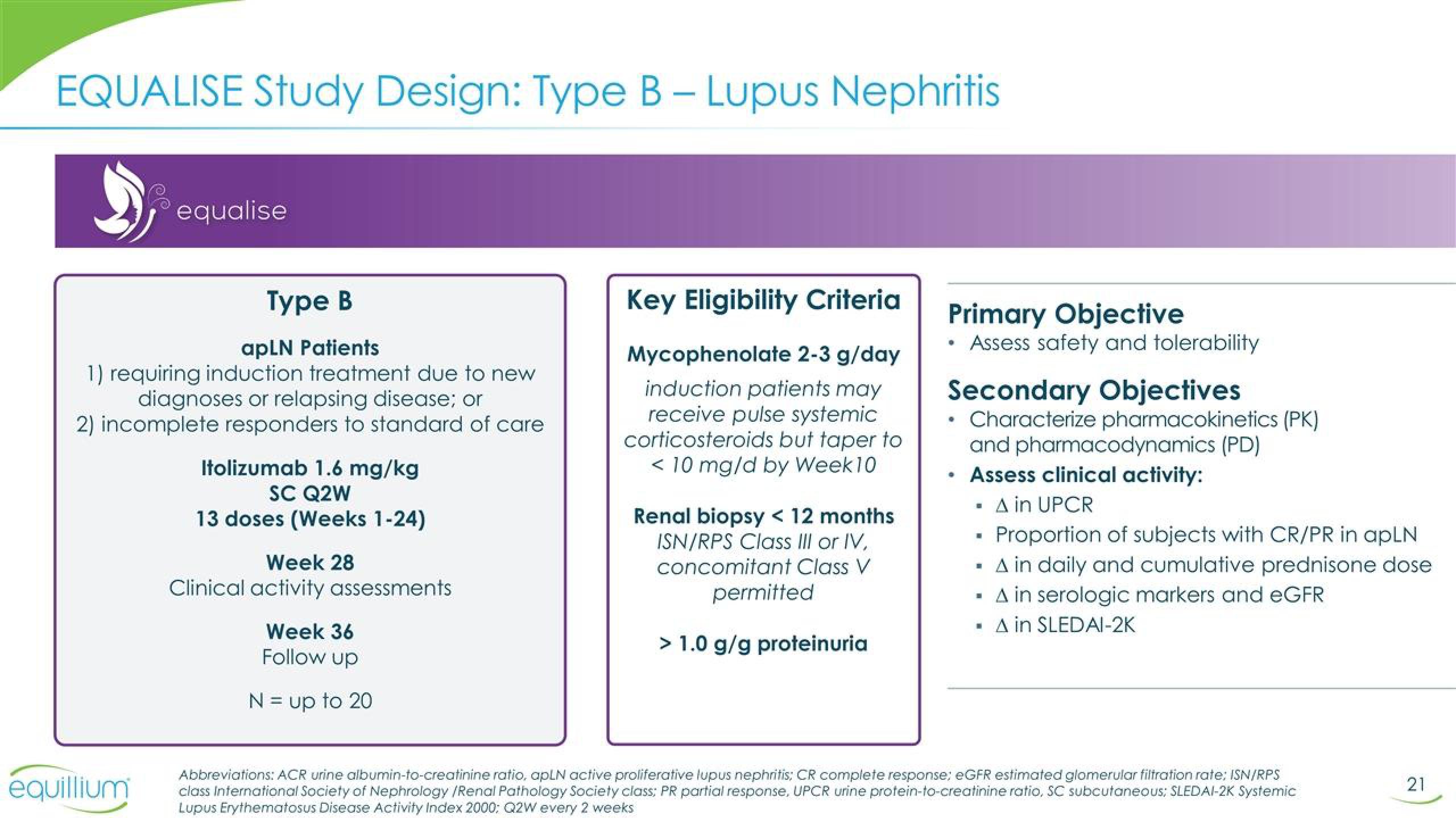
EQUALISE Study Design: Type B - Lupus Nephritis
equalise
Type B
apLN Patients
1) requiring induction treatment due to new
diagnoses or relapsing disease; or
2) incomplete responders to standard of care
equillium
Itolizumab 1.6 mg/kg
SC Q2W
13 doses (Weeks 1-24)
Week 28
Clinical activity assessments
Week 36
Follow up
N = up to 20
Key Eligibility Criteria
Mycophenolate 2-3 g/day
induction patients may
receive pulse systemic
corticosteroids but taper to
< 10 mg/d by Week 10
Renal biopsy < 12 months
ISN/RPS Class III or IV,
concomitant Class V
permitted
> 1.0 g/g proteinuria
Primary Objective
Assess safety and tolerability
.
Secondary Objectives
• Characterize pharmacokinetics (PK)
and pharmacodynamics (PD)
• Assess clinical activity:
A in UPCR
• Proportion of subjects with CR/PR in apLN
▪ A in daily and cumulative prednisone dose
▪ A in serologic markers and eGFR
A in SLEDAI-2K
■
Abbreviations: ACR urine albumin-to-creatinine ratio, apLN active proliferative lupus nephritis; CR complete response; eGFR estimated glomerular filtration rate: ISN/RPS
class International Society of Nephrology /Renal Pathology Society class: PR partial response, UPCR urine protein-to-creatinine ratio, SC subcutaneous; SLEDAI-2K Systemic
Lupus Erythematosus Disease Activity Index 2000: Q2W every 2 weeks
21View entire presentation