Neumora Therapeutics IPO Presentation Deck
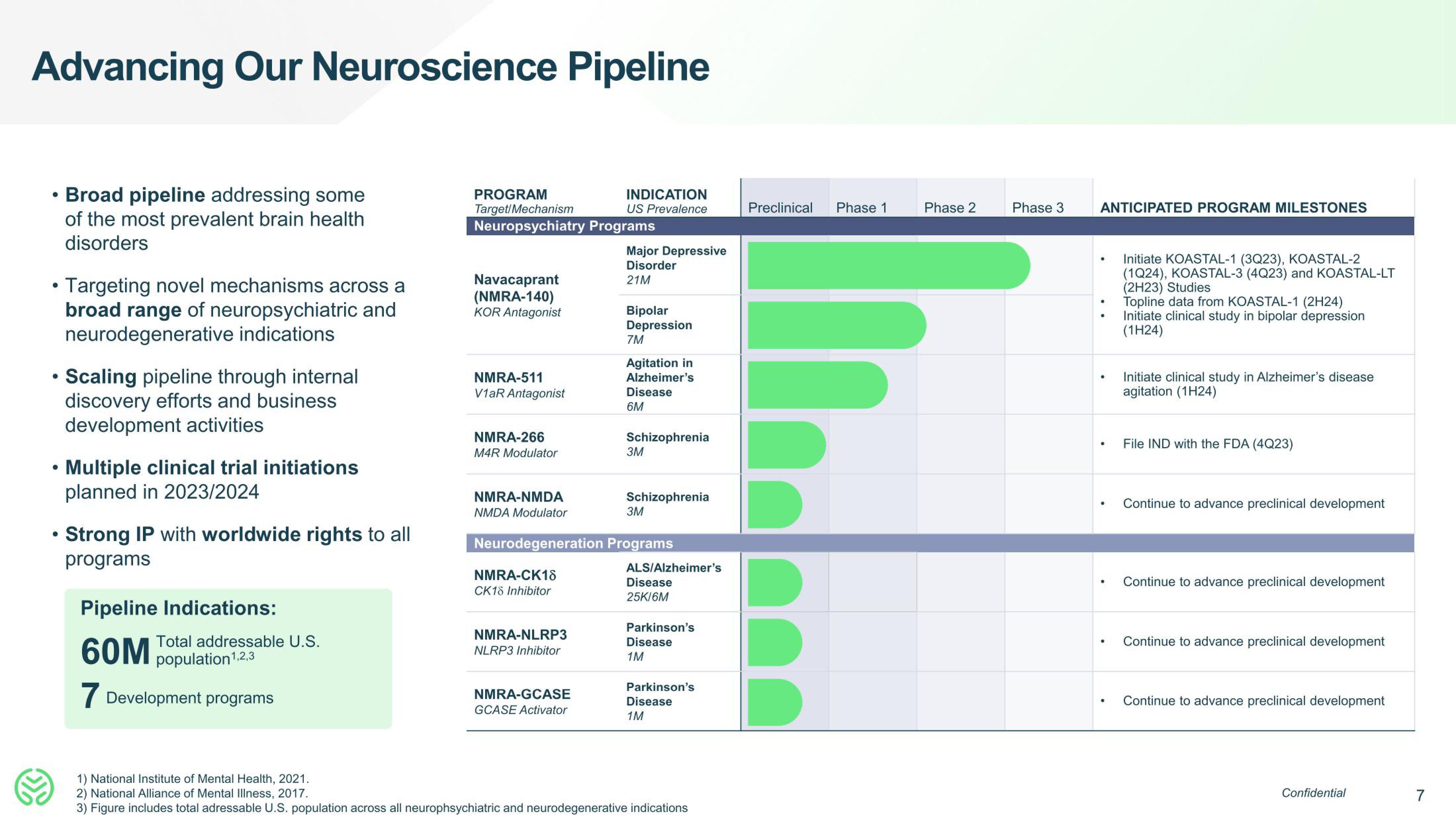
Advancing Our Neuroscience Pipeline
• Targeting novel mechanisms across a
broad range of neuropsychiatric and
neurodegenerative indications
Broad pipeline addressing some
of the most prevalent brain health
disorders
• Scaling pipeline through internal
discovery efforts and business
development activities
●
●
Multiple clinical trial initiations
planned in 2023/2024
Strong IP with worldwide rights to all
programs
Pipeline Indications:
60M
Total addressable U.S.
population 1,2,3
7 Development programs
PROGRAM
Target/Mechanism
Neuropsychiatry Programs
Navacaprant
(NMRA-140)
KOR Antagonist
NMRA-511
V1aR Antagonist
NMRA-266
M4R Modulator
NMRA-CK18
CK18 Inhibitor
INDICATION
US Prevalence
NMRA-NLRP3
NLRP3 Inhibitor
NMRA-GCASE
GCASE Activator
Major Depressive
Disorder
21M
Bipolar
Depression
7M
Agitation in
Alzheimer's
Disease
6M
NMRA-NMDA
NMDA Modulator
Neurodegeneration Programs
Schizophrenia
3M
Schizophrenia
3M
ALS/Alzheimer's
Disease
25K/6M
Parkinson's
Disease
1M
Parkinson's
Disease
1M
1) National Institute of Mental Health, 2021.
2) National Alliance of Mental Illness, 2017.
3) Figure includes total adressable U.S. population across all neurophsychiatric and neurodegenerative indications
Preclinical
Phase 1
Phase 2
Phase 3
ANTICIPATED PROGRAM MILESTONES
.
Initiate KOASTAL-1 (3Q23), KOASTAL-2
(1Q24), KOASTAL-3 (4Q23) and KOASTAL-LT
(2H23) Studies
Topline data from KOASTAL-1 (2H24)
Initiate clinical study in bipolar depression
(1H24)
Initiate clinical study in Alzheimer's disease
agitation (1H24)
File IND with the FDA (4Q23)
Continue to advance preclinical development
Continue to advance preclinical development
Continue to advance preclinical development
Continue to advance preclinical development
Confidential
7View entire presentation