BenevolentAI SPAC Presentation Deck
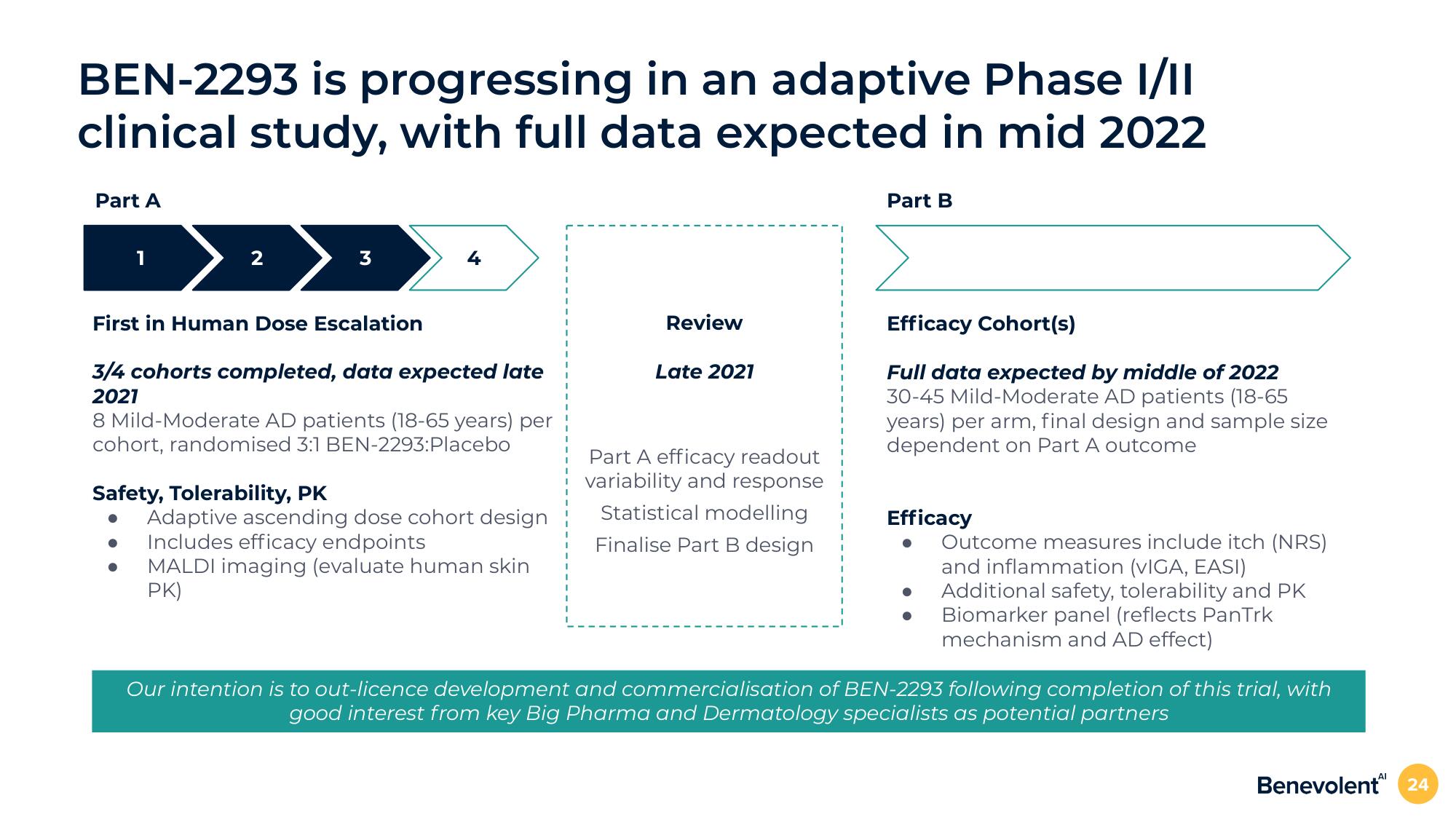
BEN-2293 is progressing in an adaptive Phase I/II
clinical study, with full data expected in mid 2022
Part A
2
3
4
First in Human Dose Escalation
3/4 cohorts completed, data expected late
2021
Safety, Tolerability, PK
8 Mild-Moderate AD patients (18-65 years) per
cohort, randomised 3:1 BEN-2293:Placebo
Adaptive ascending dose cohort design
Includes efficacy endpoints
MALDI imaging (evaluate human skin
PK)
Review
Late 2021
Part A efficacy readout
variability and response
Statistical modelling
Finalise Part B design
Part B
Efficacy Cohort(s)
Full data expected by middle of 2022
30-45 Mild-Moderate AD patients (18-65
years) per arm, final design and sample size
dependent on Part A outcome
Efficacy
Outcome measures include itch (NRS)
and inflammation (VIGA, EASI)
Additional safety, tolerability and PK
Biomarker panel (reflects PanTrk
mechanism and AD effect)
Our intention is to out-licence development and commercialisation of BEN-2293 following completion of this trial, with
good interest from key Big Pharma and Dermatology specialists as potential partners
Benevolent 24View entire presentation