Investor Presentation
28
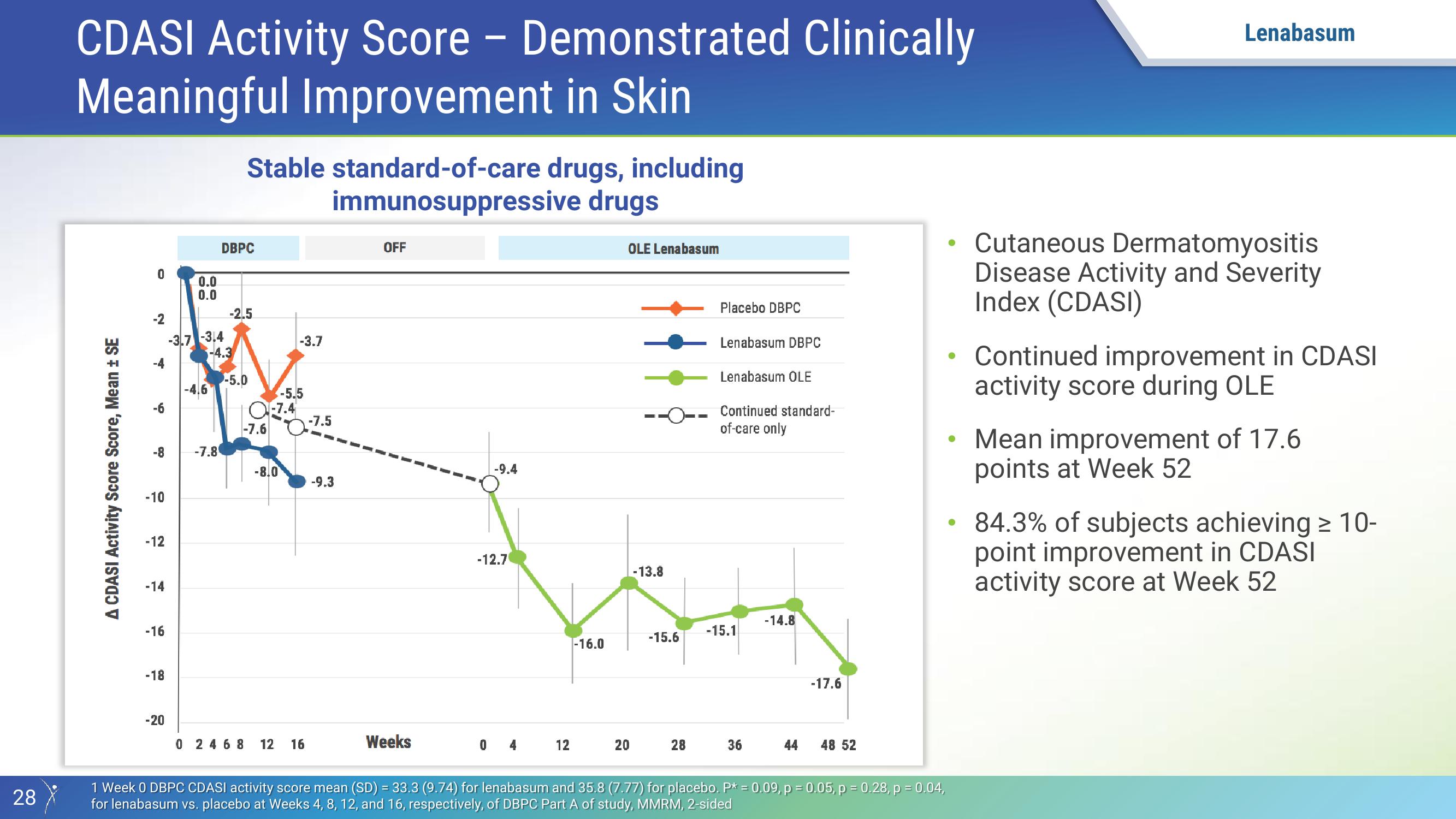
CDASI Activity Score - Demonstrated Clinically
Meaningful Improvement in Skin
A CDASI Activity Score Score, Mean ± SE
0
N
-8
-10
-12
-14
-16
-18
-20
0.0
0.0
-3.7 -3.4
16
DBPC
-7.8
Stable standard-of-care drugs, including
immunosuppressive
drugs
-4.3
-2.5
-5.0
Q-7.4
-7.6
-8.0
-3.7
-5.5
0 2468 12 16
-7.5
-9.3
OFF
Weeks
-9.4
-12.7
04
12
-16.0
OLE Lenabasum
20
-13.8
-15.6
28
Placebo DBPC
Lenabasum DBPC
Lenabasum OLE
Continued standard-
of-care only
-15.1
36
-14.8
44
-17.6
48 52
1 Week 0 DBPC CDASI activity score mean (SD) = 33.3 (9.74) for lenabasum and 35.8 (7.77) for placebo. P* = 0.09, p = 0.05, p = 0.28, p = 0.04,
for lenabasum vs. placebo at Weeks 4, 8, 12, and 16, respectively, of DBPC Part A of study, MMRM, 2-sided
●
Lenabasum
Cutaneous Dermatomyositis
Disease Activity and Severity
Index (CDASI)
Continued improvement in CDASI
activity score during OLE
• Mean improvement of 17.6
points at Week 52
84.3% of subjects achieving ≥ 10-
point improvement in CDASI
activity score at Week 52View entire presentation