BioNTech Investor Day Presentation Deck
Fix Vac IBNT111
Durable responses in a Phase 1/2 trial in advanced CPI-experienced melanoma
Article
An RNA vaccine drives immunity in
checkpoint-inhibitor-treated melanoma
https://doi.org/10.1038/s41586-020-2537-9 Ugur Sahin¹2,3,4, Petra Oehm', Evelyna Derhovanessian, Robert A. Jabulowsky'.
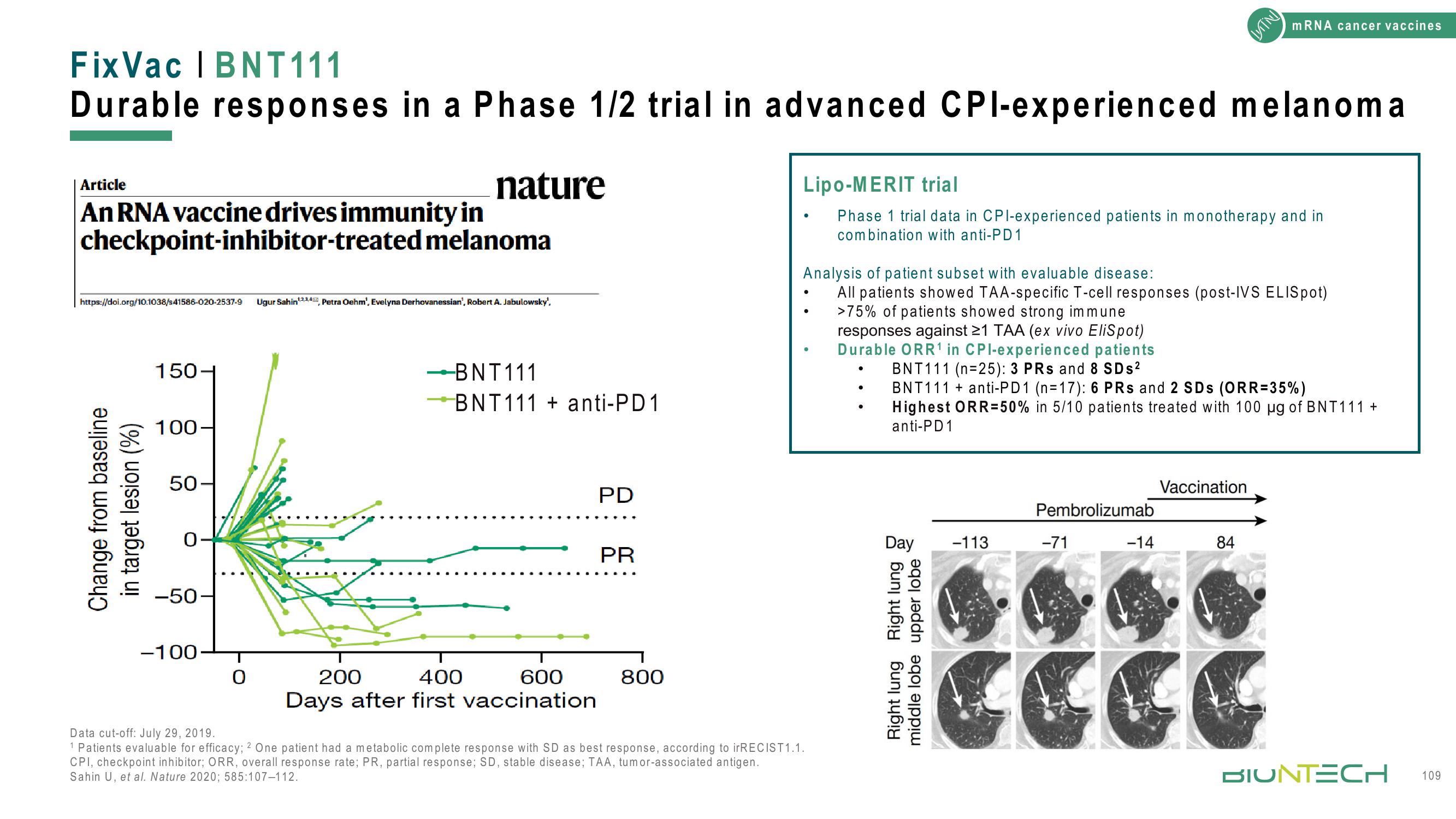
Change from baseline
in target lesion (%)
150
100-
50-
0
-50-
nature
-100
0
-BNT111
BNT111+ anti-PD1
200
400
600
Days after first vaccination
PD
PR
800
Lipo-MERIT trial
Phase 1 trial data in CPI-experienced patients in monotherapy and in
combination with anti-PD1
Data cut-off: July 29, 2019.
¹ Patients evaluable for efficacy; 2 One patient had a metabolic complete response with SD as best response, according to irRECIST1.1.
CPI, checkpoint inhibitor; ORR, overall response rate; PR, partial response; SD, stable disease; TAA, tumor-associated antigen.
Sahin U, et al. Nature 2020; 585:107-112.
Analysis of patient subset with evaluable disease:
All patients showed TAA-specific T-cell responses (post-IVS ELISpot)
>75% of patients showed strong immune
responses against 21 TAA (ex vivo EliSpot)
Durable ORR¹ in CPI-experienced patients
BNT111 (n=25): 3 PRs and 8 SDs²
BNT111+ anti-PD1 (n=17): 6 PRs and 2 SDs (ORR=35%)
Highest ORR=50% in 5/10 patients treated with 100 µg of BNT111 +
anti-PD 1
●
Day
Right lung Right lung
middle lobe upper lobe
-113
Pembrolizumab
-71
NUM
-14
mRNA cancer vaccines
Vaccination
84
BIUNTECH
109View entire presentation