Ocuphire Pharma Results
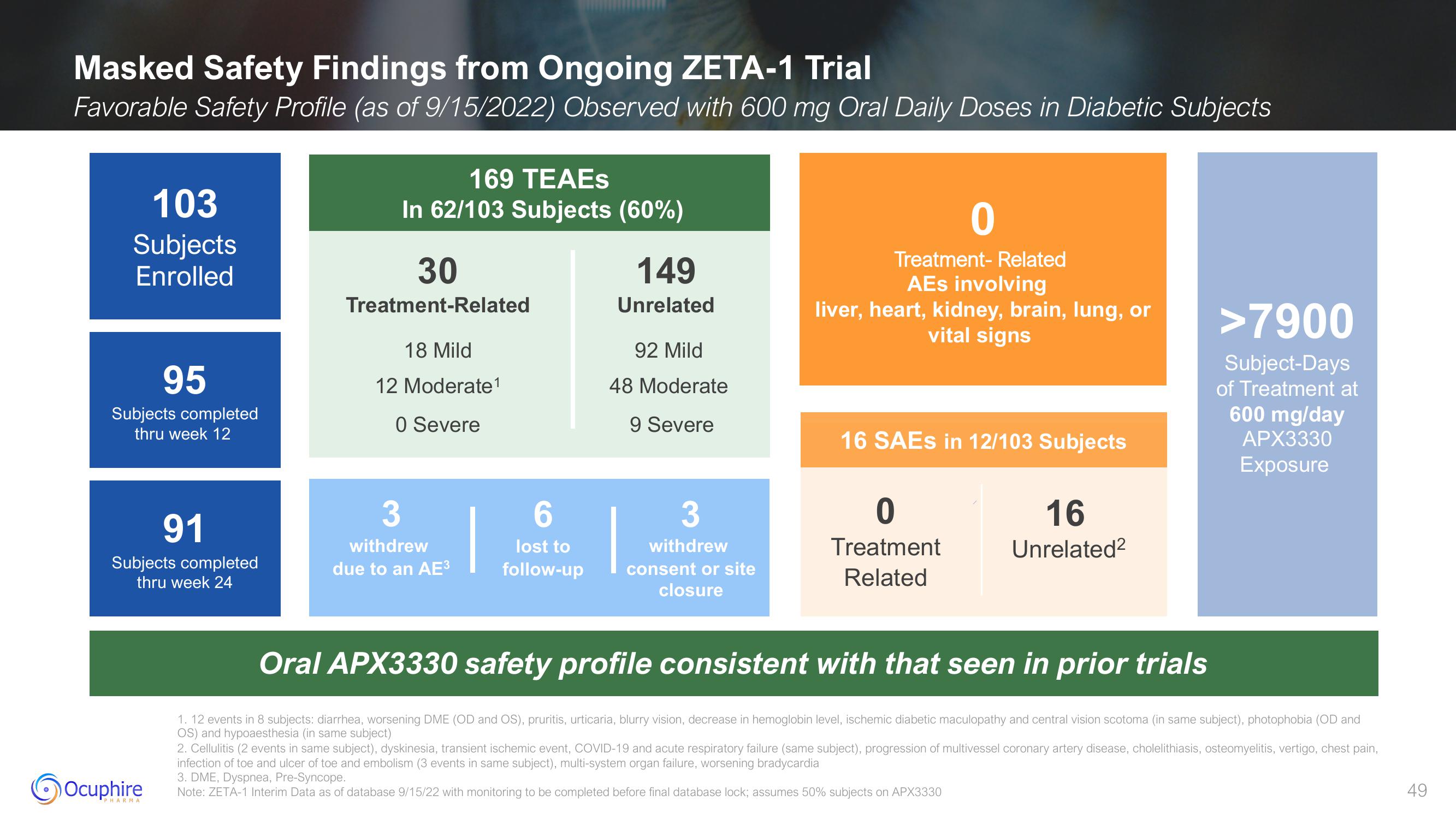
Masked Safety Findings from Ongoing ZETA-1 Trial
Favorable Safety Profile (as of 9/15/2022) Observed with 600 mg Oral Daily Doses in Diabetic Subjects
103
Subjects
Enrolled
95
Subjects completed
thru week 12
91
Subjects completed
thru week 24
Ocuphire
PHARMA
169 TEAES
In 62/103 Subjects (60%)
30
Treatment-Related
18 Mild
12 Moderate¹
0 Severe
3
withdrew
due to an AE³
6
lost to
follow-up
149
Unrelated
92 Mild
48 Moderate
9 Severe
3
withdrew
consent or site
closure
0
Treatment- Related
AEs involving
liver, heart, kidney, brain, lung, or
vital signs
16 SAES in 12/103 Subjects
0
Treatment
Related
16
Unrelated²
>7900
Subject-Days
of Treatment at
600 mg/day
APX3330
Exposure
Oral APX3330 safety profile consistent with that seen in prior trials
1. 12 events in 8 subjects: diarrhea, worsening DME (OD and OS), pruritis, urticaria, blurry vision, decrease in hemoglobin level, ischemic diabetic maculopathy and central vision scotoma (in same subject), photophobia (OD and
OS) and hypoaesthesia (in same subject)
2. Cellulitis (2 events in same subject), dyskinesia, transient ischemic event, COVID-19 and acute respiratory failure (same subject), progression of multivessel coronary artery disease, cholelithiasis, osteomyelitis, vertigo, chest pain,
infection of toe and ulcer of toe and embolism (3 events in same subject), multi-system organ failure, worsening bradycardia
3. DME, Dyspnea, Pre-Syncope.
Note: ZETA-1 Interim Data as of database 9/15/22 with monitoring to be completed before final database lock; assumes 50% subjects on APX3330
49View entire presentation