BioNTech Results Presentation Deck
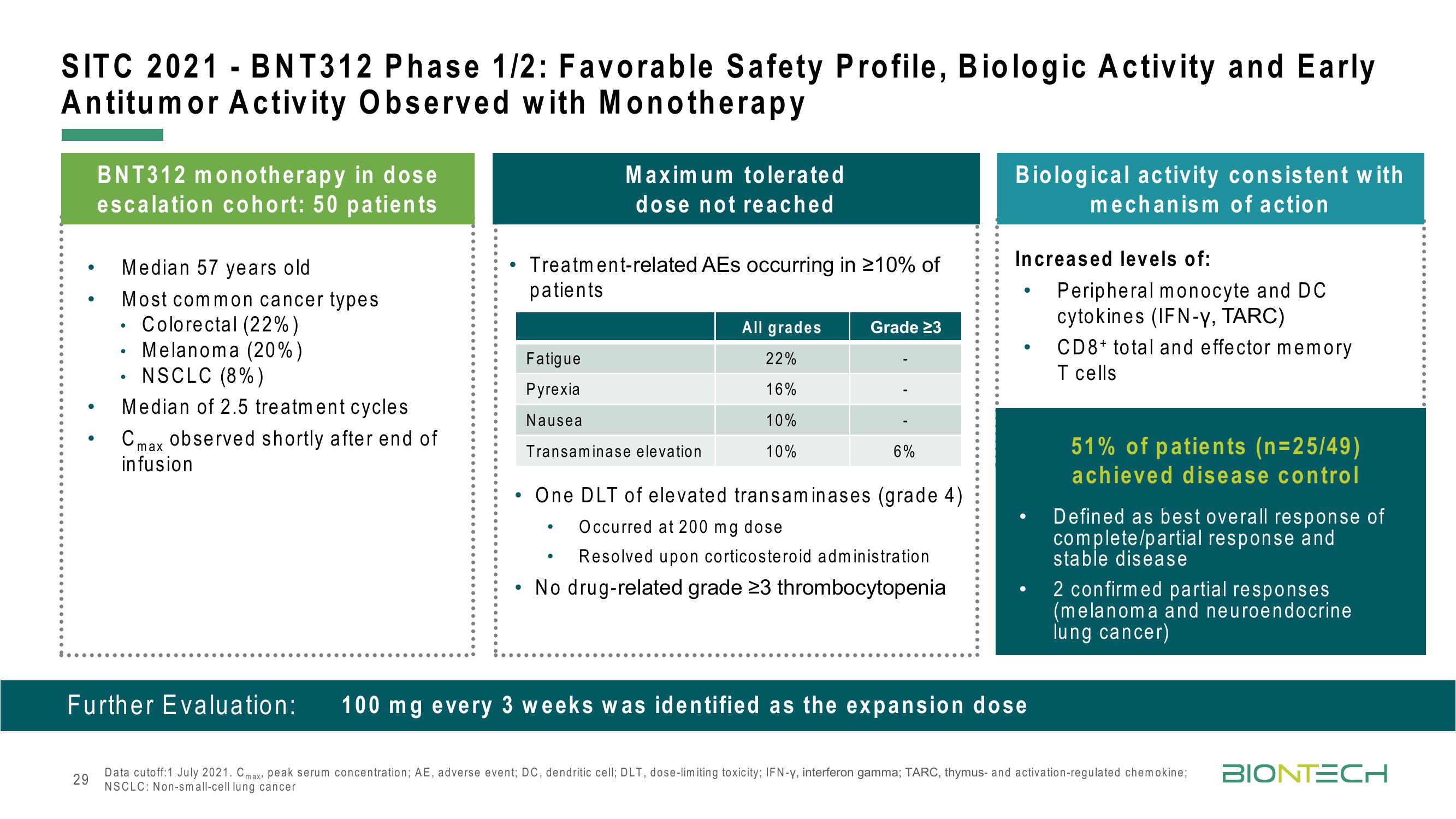
SITC 2021 - BNT312 Phase 1/2: Favorable Safety Profile, Biologic Activity and Early
Antitumor Activity Observed with Monotherapy
●
●
●
BNT312 monotherapy in dose
escalation cohort: 50 patients
29
Median 57 years old
Most common cancer types
. Colorectal (22%)
Melanoma (20%)
NSCLC (8%)
Median of 2.5 treatment cycles
C max observed shortly after end of
infusion
●
●
●
Treatment-related AEs occurring in ≥10% of
patients
Fatigue
Pyrexia
Nausea
Maximum tolerated
dose not reached
Transaminase elevation
●
All grades
22%
16%
10%
10%
Grade 23
One DLT of elevated transaminases (grade 4)
Occurred at 200 mg dose
Resolved upon corticosteroid administration
• No drug-related grade 23 thrombocytopenia
6%
Biological activity consistent with
mechanism of action
Increased levels of:
●
Further Evaluation: 100 mg every 3 weeks was identified as the expansion dose
Peripheral monocyte and DC
cytokines (IFN-Y, TARC)
CD8+ total and effector memory
T cells
51% of patients (n=25/49)
achieved disease control
Defined as best overall response of
complete/partial response and
stable disease
2 confirmed partial responses
(melanoma and neuroendocrine
lung cancer)
Data cutoff:1 July 2021. Cmax, peak serum concentration; AE, adverse event; DC, dendritic cell; DLT, dose-limiting toxicity; IFN-y, interferon gamma; TARC, thymus- and activation-regulated chemokine;
NSCLC: Non-small-cell lung cancer
BIONTECHView entire presentation