AstraZeneca Investor Day Presentation Deck
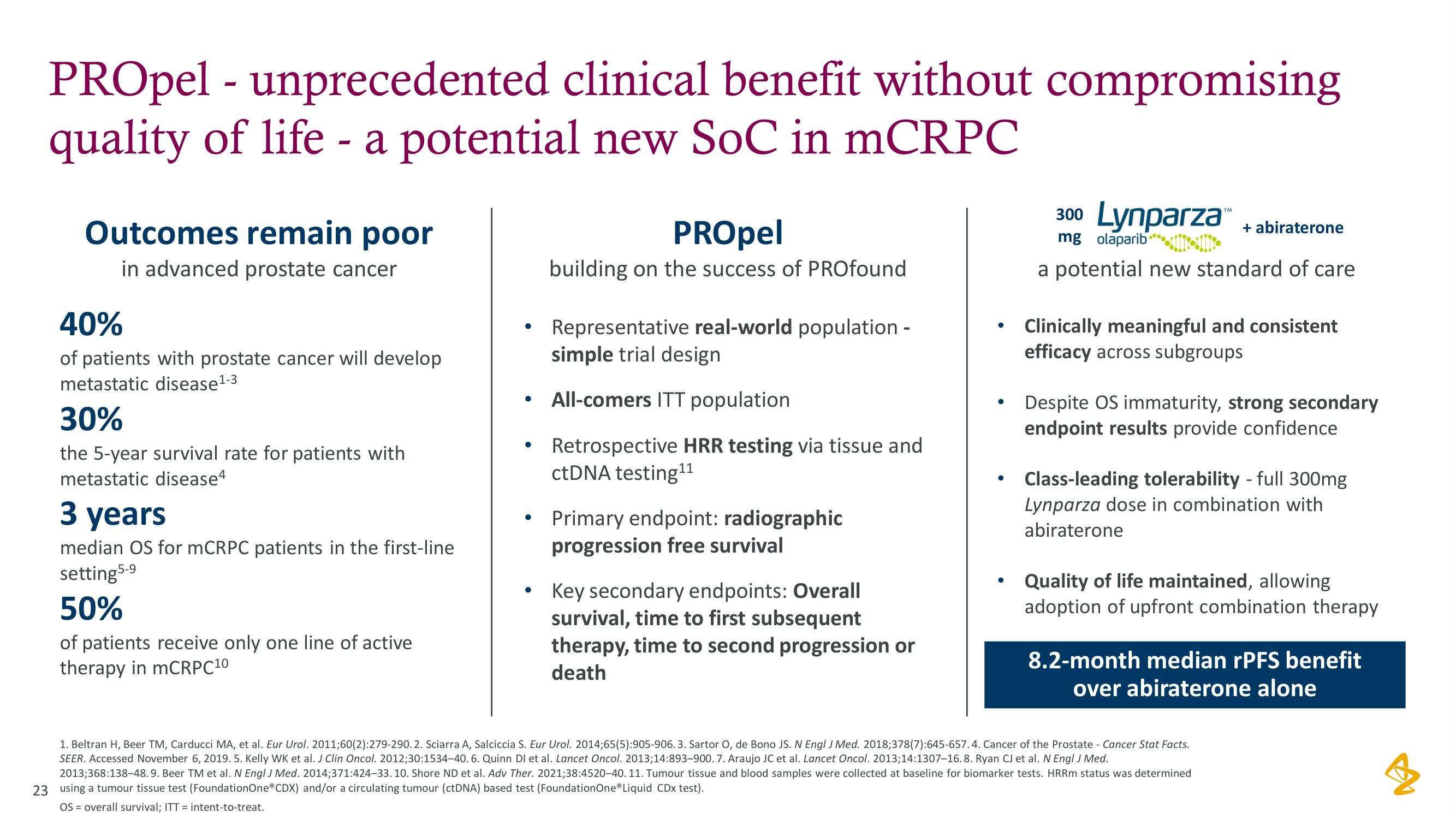
PROpel - unprecedented clinical benefit without compromising
quality of life - a potential new SoC in mCRPC
Outcomes remain poor
in advanced prostate cancer
40%
of patients with prostate cancer will develop
metastatic disease ¹-3
30%
the 5-year survival rate for patients with
metastatic disease4
3 years
median OS for mCRPC patients in the first-line
setting 5-9
50%
of patients receive only one line of active
therapy in mCRPC10
●
●
●
●
PROpel
building on the success of PROfound
Representative real-world population -
simple trial design
All-comers ITT population
Retrospective HRR testing via tissue and
ctDNA testing¹¹
Primary endpoint: radiographic
progression free survival
Key secondary endpoints: Overall
survival, time to first subsequent
therapy, time to second progression or
death
●
300 Lynparza™
+ abiraterone
mg olaparib
a potential new standard of care
Clinically meaningful and consistent
efficacy across subgroups
Despite OS immaturity, strong secondary
endpoint results provide confidence
Class-leading tolerability - full 300mg
Lynparza dose in combination with
abiraterone
Quality of life maintained, allowing
adoption of upfront combination therapy
8.2-month median rPFS benefit
over abiraterone alone
1. Beltran H, Beer TM, Carducci MA, et al. Eur Urol. 2011;60(2):279-290. 2. Sciarra A, Salciccia S. Eur Urol. 2014;65(5):905-906. 3. Sartor O, de Bono JS. N Engl J Med. 2018;378(7):645-657.4. Cancer of the Prostate - Cancer Stat Facts.
SEER. Accessed November 6, 2019. 5. Kelly WK et al. J Clin Oncol. 2012;30:1534-40. 6. Quinn DI et al. Lancet Oncol. 2013;14:893-900. 7. Araujo JC et al. Lancet Oncol. 2013;14:1307-16.8. Ryan CJ et al. N Engl J Med.
2013;368:138-48.9. Beer TM et al. N Engl J Med. 2014;371:424-33. 10. Shore ND et al. Adv Ther. 2021;38:4520-40. 11. Tumour tissue and blood samples were collected at baseline for biomarker tests. HRRm status was determined
23 using a tumour tissue test (Foundation One® CDX) and/or a circulating tumour (ctDNA) based test (FoundationOneⓇLiquid CDx test).
OS = overall survival; ITT = intent-to-treat.View entire presentation