Immix Biopharma Investor Presentation Deck
Sizable U.S. Relapsed/Refractory AL Amyloidosis Patient Population
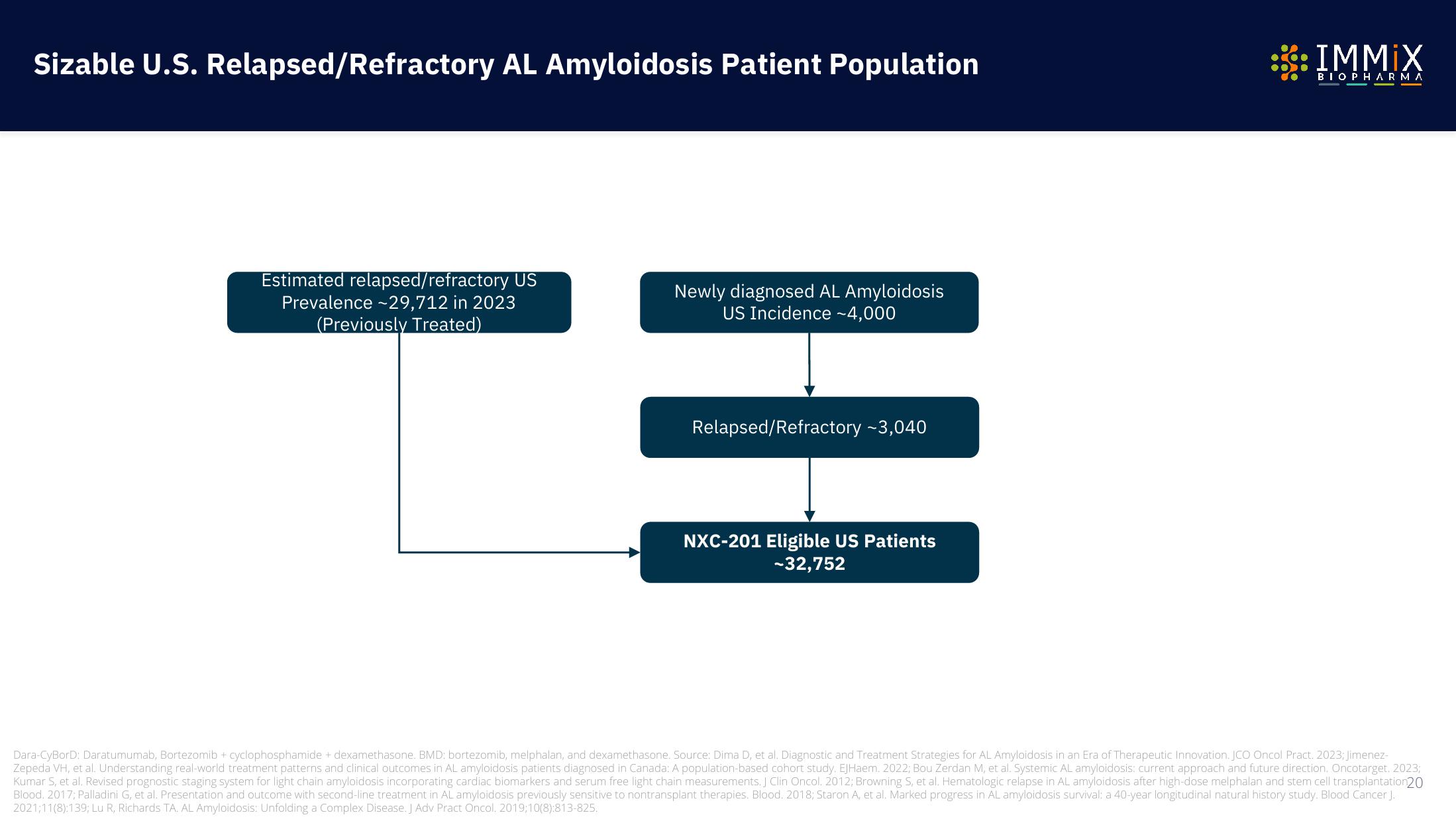
Estimated relapsed/refractory US
Prevalence ~29,712 in 2023
(Previously Treated)
Newly diagnosed AL Amyloidosis
US Incidence ~4,000
Relapsed/Refractory -3,040
NXC-201 Eligible US Patients
-32,752
●●●
S
IMMIX
BIOPHARMA
Dara-CyBorD: Daratumumab, Bortezomib + cyclophosphamide + dexamethasone. BMD: bortezomib, melphalan, and dexamethasone. Source: Dima D, et al. Diagnostic and Treatment Strategies for AL Amyloidosis in an Era of Therapeutic Innovation. JCO Oncol Pract. 2023; Jimenez-
Zepeda VH, et al. Understanding real-world treatment patterns and clinical outcomes in AL amyloidosis patients diagnosed in Canada: A population-based cohort study. EJHaem. 2022; Bou Zerdan M, et al. Systemic AL amyloidosis: current approach and future direction. Oncotarget. 2023;
Kumar S, et al. Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012; Browning S, et al. Hematologic relapse in AL amyloidosis after high-dose melphalan and stem cell transplantation20
Blood. 2017; Palladini G, et al. Presentation and outcome with second-line treatment in AL amyloidosis previously sensitive to nontransplant therapies. Blood. 2018; Staron A, et al. Marked progress in AL amyloidosis survival: a 40-year longitudinal natural history study. Blood Cancer J.
2021;11(8):139; Lu R, Richards TA. AL Amyloidosis: Unfolding a Complex Disease. J Adv Pract Oncol. 2019;10(8):813-825.View entire presentation