ATAI Investor Day Presentation Deck
Development program overview: lead compounds, lead indications and stage of
development
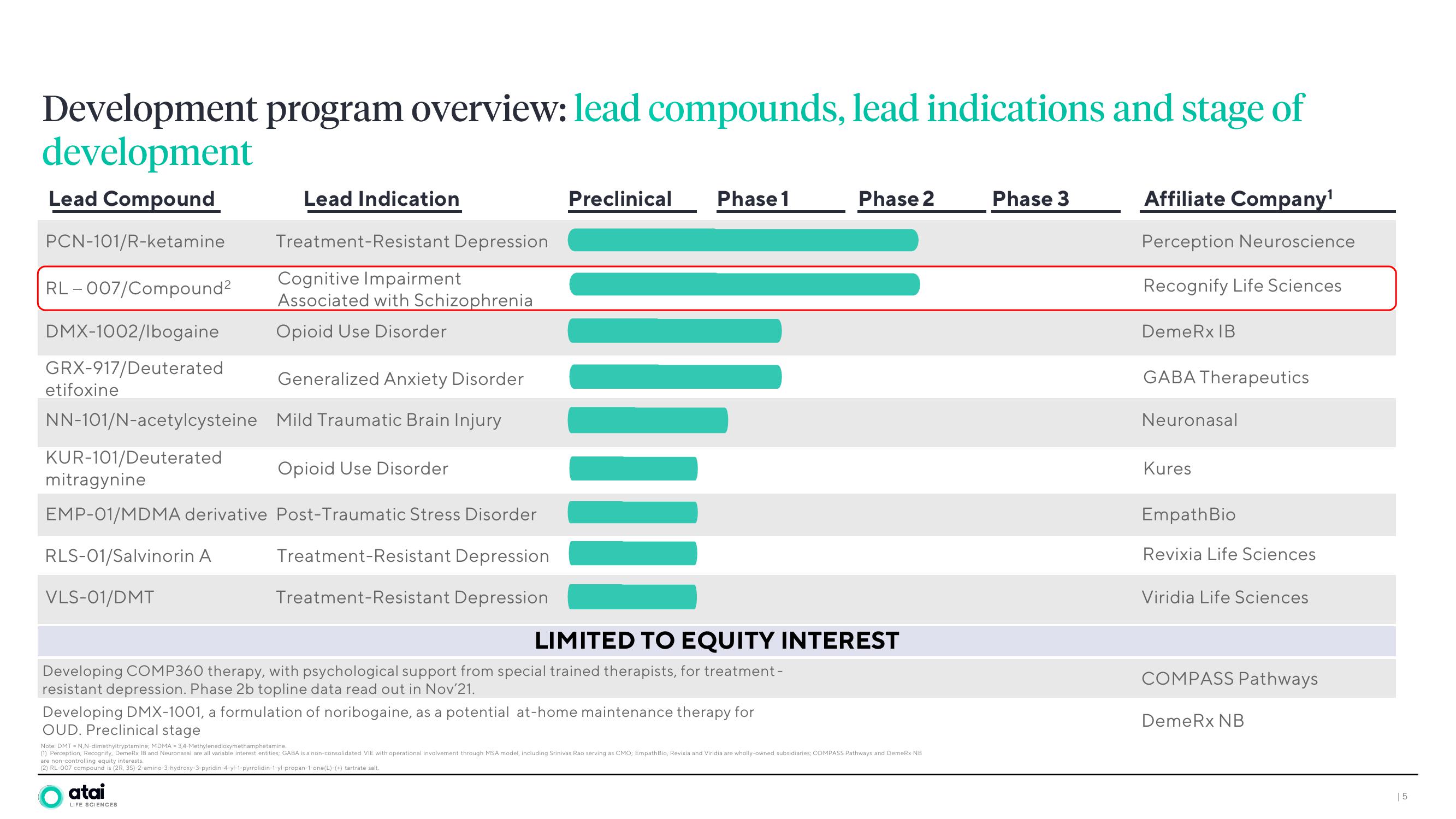
Lead Compound
PCN-101/R-ketamine
RL - 007/Compound²
DMX-1002/lbogaine
GRX-917/Deuterated
etifoxine
NN-101/N-acetylcysteine
KUR-101/Deuterated
Generalized Anxiety Disorder
Mild Traumatic Brain Injury
Opioid Use Disorder
mitragynine
EMP-01/MDMA derivative Post-Traumatic Stress Disorder
RLS-01/Salvinorin A
VLS-01/DMT
Lead Indication
Treatment-Resistant Depression
atai
LIFE SCIENCES
Cognitive Impairment
Associated with Schizophrenia
Opioid Use Disorder
Preclinical
Treatment-Resistant Depression
Treatment-Resistant Depression
Phase 1
Phase 2
E
LIMITED TO EQUITY INTEREST
Developing COMP360 therapy, with psychological support from special trained therapists, for treatment -
resistant depression. Phase 2b topline data read out in Nov'21.
Developing DMX-1001, a formulation of noribogaine, as a potential at-home maintenance therapy for
OUD. Preclinical stage
Note: DMT = N,N-dimethyltryptamine; MDMA = 3,4-Methylenedioxymethamphetamine.
(1) Perception, Recognify, DemeRx IB and Neuronasal are all variable interest entities; GABA is a non-consolidated VIE with operational involvement through MSA model, including Srinivas Rao serving as CMO; EmpathBio, Revixia and Viridia are wholly-owned subsidiaries; COMPASS Pathways and DemeRx NB
are non-controlling equity interests.
(2) RL-007 compound is (2R, 3S)-2-amino-3-hydroxy-3-pyridin-4-yl-1-pyrrolidin-1-yl-propan-1-one(L)-(+) tartrate salt.
Phase 3
Affiliate Company¹
Perception Neuroscience
Recognify Life Sciences
DemeRx IB
GABA Therapeutics
Neuronasal
Kures
Empath Bio
Revixia Life Sciences
Viridia Life Sciences
COMPASS Pathways
DemeRx NB
15View entire presentation