AstraZeneca Results Presentation Deck
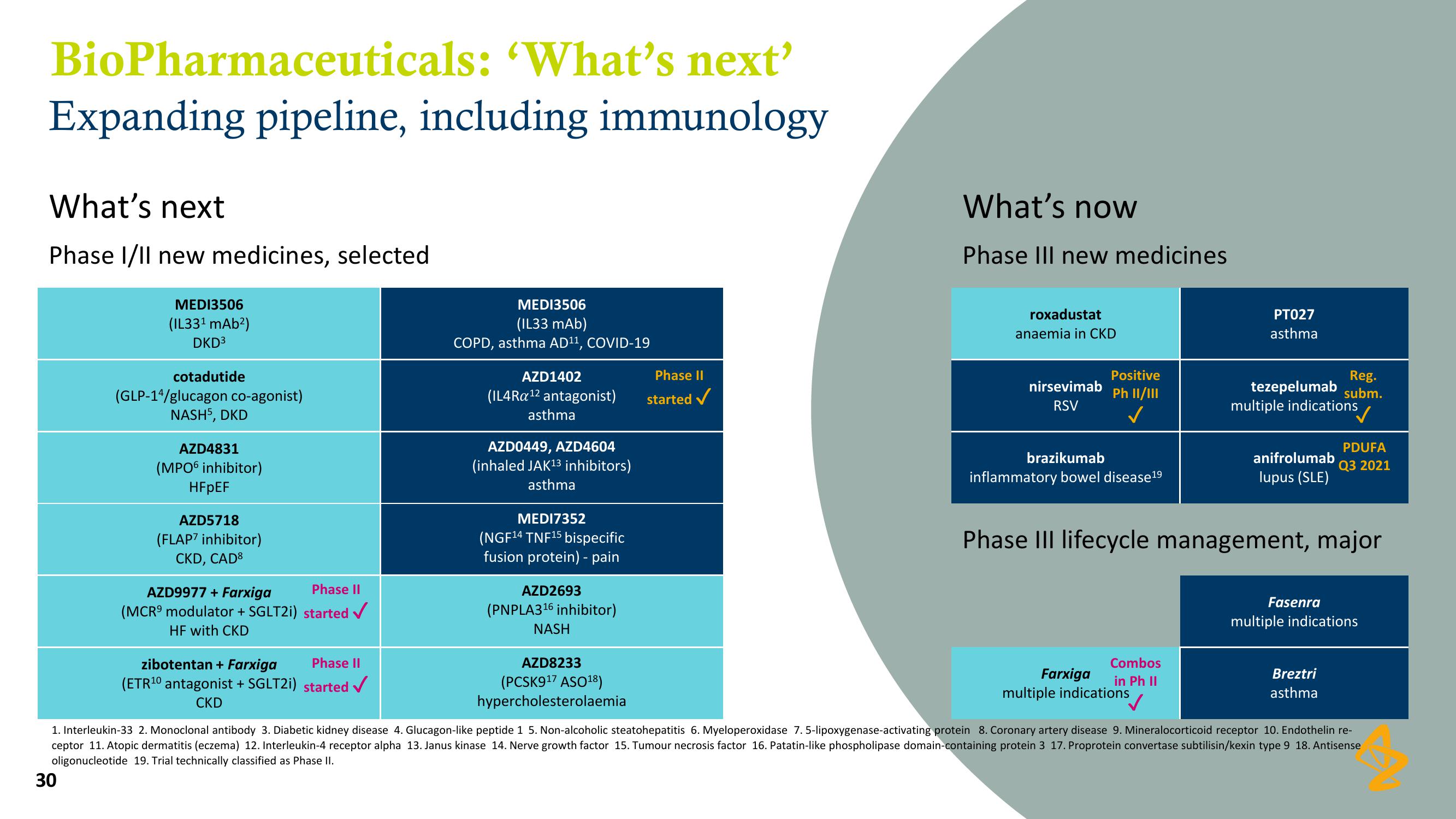
BioPharmaceuticals:
'What's next'
Expanding pipeline, including immunology
What's next
Phase I/II new medicines, selected
MEDI3506
(IL33¹ mAb²)
DKD³
30
cotadutide
(GLP-14/glucagon co-agonist)
NASH5, DKD
AZD4831
(MPO6 inhibitor)
HFpEF
AZD5718
(FLAP7 inhibitor)
CKD, CAD8
AZD9977 + Farxiga
(MCR modulator + SGLT2i)
HF with CKD
Phase II
started ✓
zibotentan + Farxiga
Phase II
(ETR¹0 antagonist +SGLT2i) started ✓
CKD
MEDI3506
(IL33 mAb)
COPD, asthma AD¹¹, COVID-19
AZD1402
(IL4Ra ¹2 antagonist)
asthma
AZD0449, AZD4604
(inhaled JAK¹3 inhibitors)
asthma
MEDI7352
(NGF¹4 TNF¹5 bispecific
fusion protein) - pain
AZD2693
(PNPLA3¹6 inhibitor)
NASH
AZD8233
(PCSK9¹7 ASO¹8)
hypercholesterolaemia
Phase II
started ✓
What's now
Phase III new medicines
roxadustat
anaemia in CKD
nirsevimab
RSV
Positive
Ph II/III
✓
brazikumab
inflammatory bowel disease19
PT027
asthma
Combos
Farxiga
in Ph II
multiple indications
Reg.
tezepelumab subm.
multiple indications
anifrolumab
lupus (SLE)
Phase III lifecycle management, major
Fasenra
PDUFA
Q3 2021
multiple indications
Breztri
asthma
1. Interleukin-33 2. Monoclonal antibody 3. Diabetic kidney disease 4. Glucagon-like peptide 1 5. Non-alcoholic steatohepatitis 6. Myeloperoxidase 7.5-lipoxygenase-activating protein 8. Coronary artery disease 9. Mineralocorticoid receptor 10. Endothelin re-
ceptor 11. Atopic dermatitis (eczema) 12. Interleukin-4 receptor alpha 13. Janus kinase 14. Nerve growth factor 15. Tumour necrosis factor 16. Patatin-like phospholipase domain-containing protein 3 17. Proprotein convertase subtilisin/kexin type 9 18. Antisense
oligonucleotide 19. Trial technically classified as Phase II.View entire presentation