BioNTech Results Presentation Deck
Clinical Data Support Label Extension of BNT162b2 to Children 5 to 11 Years of Age¹
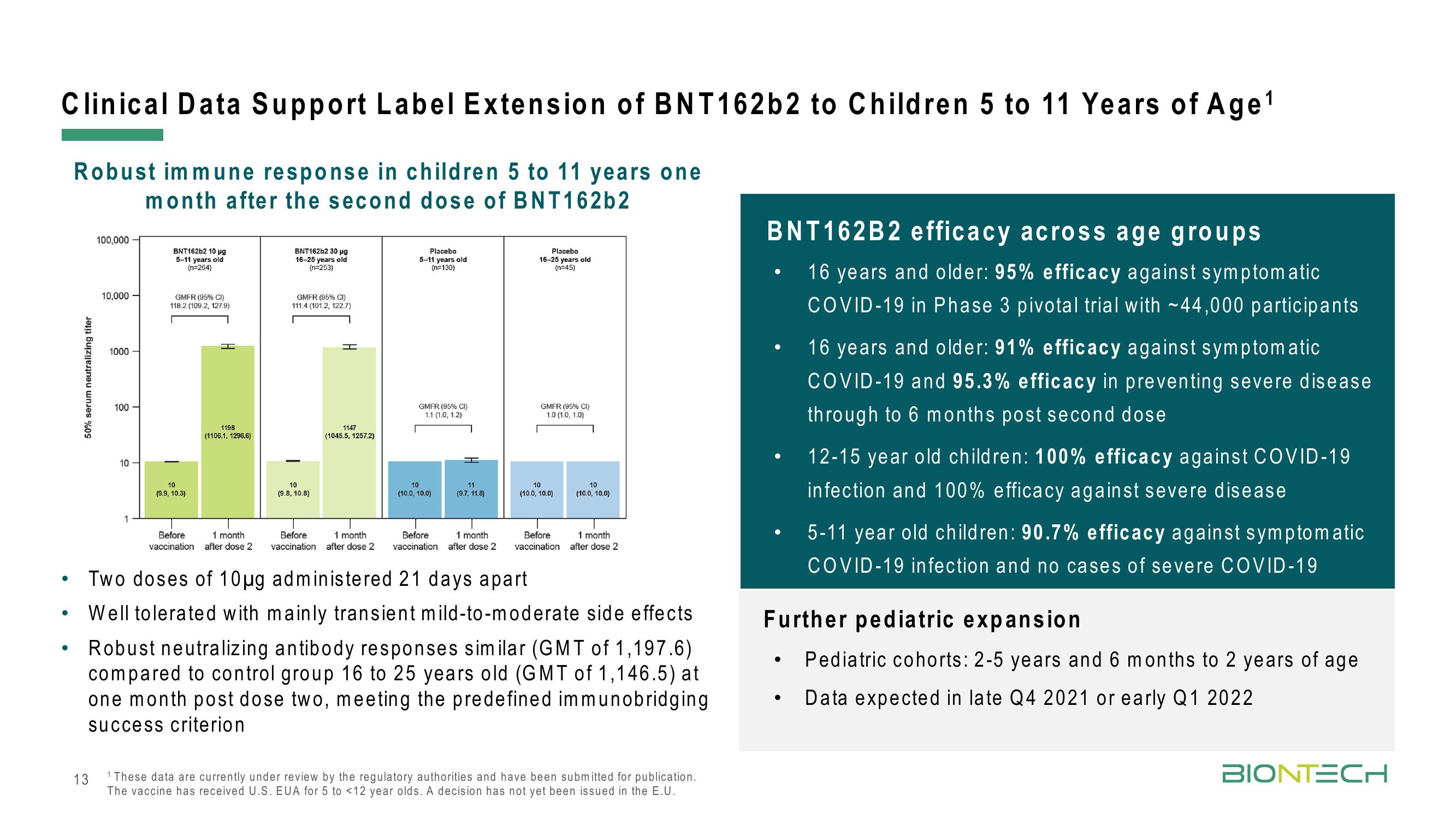
Robust immune response in children 5 to 11 years one
month after the second dose of BNT162b2
●
●
50% serum neutralizing titer
100,000
10,000
1000
100
10
BNT162b2 10 µg
5-11 years old
(n=264)
GMFR (95% CI)
118.2 (109.2, 127.9)
10
(9.9, 10.3)
I
1198
(1106.1, 1296.6)
Before 1 month
vaccination after dose 2
BNT162b2 30 μg
16-25 years old
(n=253)
GMFR (95% CI)
111.4 (101.2, 122.7)
10
(9.8, 10.8)
T
1147
(1045.5, 1257.2)
Placebo
5-11 years old
(n=130)
GMFR (95% CI)
1.1 (1.0, 1.2)
10
(10.0, 10.0)
11
(9.7, 11.8)
1 month
Before
1 month
Before
vaccination after dose 2 vaccination after dose 2
Placebo
16-25 years old
(n=45)
GMFR (95% CI)
1.0 (1.0, 1.0)
10
(10.0, 10.0)
10
(10.0, 10.0)
1 month
Before
vaccination after dose 2
Two doses of 10µg administered 21 days apart
Well tolerated with mainly transient mild-to-moderate side effects
Robust neutralizing antibody responses similar (GMT of 1,197.6)
compared to control group 16 to 25 years old (GMT of 1,146.5) at
one month post dose two, meeting the predefined immunobridging
success criterion
13
1 These data are currently under review by the regulatory authorities and have been submitted for publication.
The vaccine has received U.S. EUA for 5 to <12 year olds. A decision has not yet been issued in the E.U.
BNT162B2 efficacy across age groups
16 years and older: 95% efficacy against symptomatic
COVID-19 in Phase 3 pivotal trial with ~44,000 participants
16 years and older: 91% efficacy against symptomatic
COVID-19 and 95.3% efficacy in preventing severe disease
through to 6 months post second dose
●
●
12-15 year old children: 100% efficacy against COVID-19
infection and 100% efficacy against severe disease
Further pediatric expansion
Pediatric cohorts: 2-5 years and 6 months to 2 years of age
Data expected in late Q4 2021 or early Q1 2022
●
5-11 year old children: 90.7% efficacy against symptomatic
COVID-19 infection and no cases of severe COVID-19
BIONTECHView entire presentation