BenevolentAI Investor Day Presentation Deck
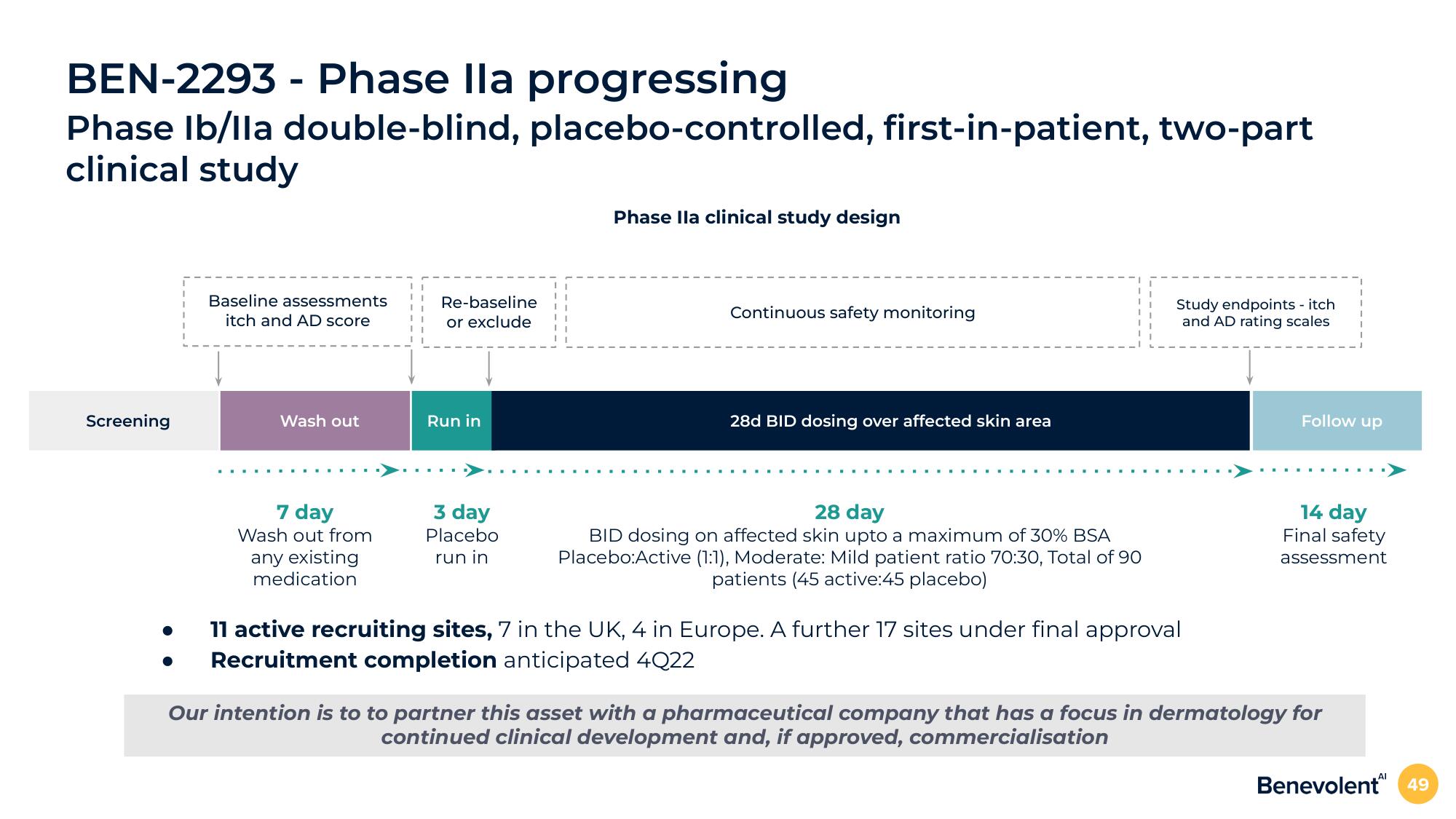
BEN-2293-Phase Ila progressing
Phase Ib/Ila double-blind, placebo-controlled, first-in-patient, two-part
clinical study
Screening
Baseline assessments
itch and AD score
Wash out
7 day
Wash out from
any existing
medication
Re-baseline
or exclude
Run in
3 day
Placebo
run in
Phase Ila clinical study design
Continuous safety monitoring
28d BID dosing over affected skin area
28 day
BID dosing on affected skin upto a maximum of 30% BSA
Placebo:Active (1:1), Moderate: Mild patient ratio 70:30, Total of 90
patients (45 active:45 placebo)
Study endpoints - itch
and AD rating scales
11 active recruiting sites, 7 in the UK, 4 in Europe. A further 17 sites under final approval
Recruitment completion anticipated 4Q22
Follow up
14 day
Final safety
assessment
Our intention is to to partner this asset with a pharmaceutical company that has a focus in dermatology for
continued clinical development and, if approved, commercialisation
Benevolent 49View entire presentation