AstraZeneca Results Presentation Deck
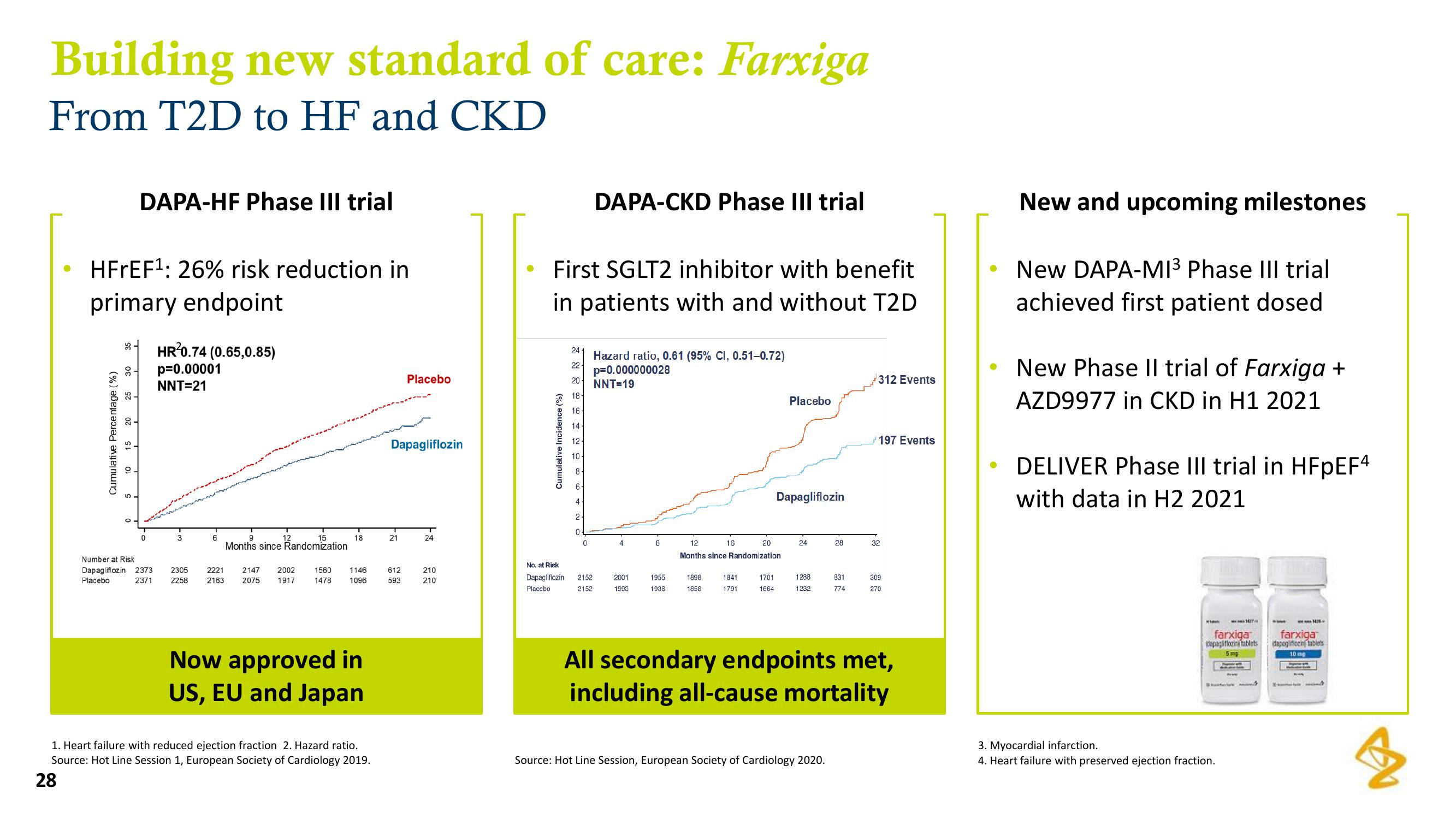
Building new standard of care: Farxiga
From T2D to HF and CKD
HFrEF¹: 26% risk reduction in
primary endpoint
Cumulative Percentage (%)
DAPA-HF Phase III trial
3-
Number at Risk
Dapagliflozin 2373
Placebo
2371
HR²0.74 (0.65,0.85)
p=0.00001
NNT=21
2305
2258
2221
2163
9
15
Months since Randomization
12
2147
2002
1917
2075
1560
1478
18
1146
1096
Now approved in
US, EU and Japan
1. Heart failure with reduced ejection fraction 2. Hazard ratio.
Source: Hot Line Session 1, European Society of Cardiology 2019.
28
Dapagliflozin
21
Placebo
612
593
24
210
210
[.
First SGLT2 inhibitor with benefit
in patients with and without T2D
Cumulative Incidence (%)
No. at Risk
Dapagliflozin
Placebo
24
22
20
18
16-
14
12
10
8
6
4
2
0
0
DAPA-CKD Phase III trial
Hazard ratio, 0.61 (95% CI, 0.51-0.72)
p=0.000000028
NNT=19
2152
2152
2001
1993
8
1955
1936
16
1898
1858
12
Months since Randomization
20
1841
1791
1701
1664
Placebo
Dapagliflozin
24
1288
1232
28
Source: Hot Line Session, European Society of Cardiology 2020.
831
774
312 Events
197 Events
32
309
270
All secondary endpoints met,
including all-cause mortality
New and upcoming milestones
New DAPA-MI³ Phase III trial
achieved first patient dosed
New Phase II trial of Farxiga +
AZD9977 in CKD in H1 2021
DELIVER Phase III trial in HFpEF4
with data in H2 2021
farxiga
dapagliflozin tablets
5mg
3. Myocardial infarction.
4. Heart failure with preserved ejection fraction.
3077-
ww
1428
farxiga
dapegifozin tablets
10 mg
3View entire presentation