Ocuphire Pharma Investor Updates
P
Percent of Subjects (%)
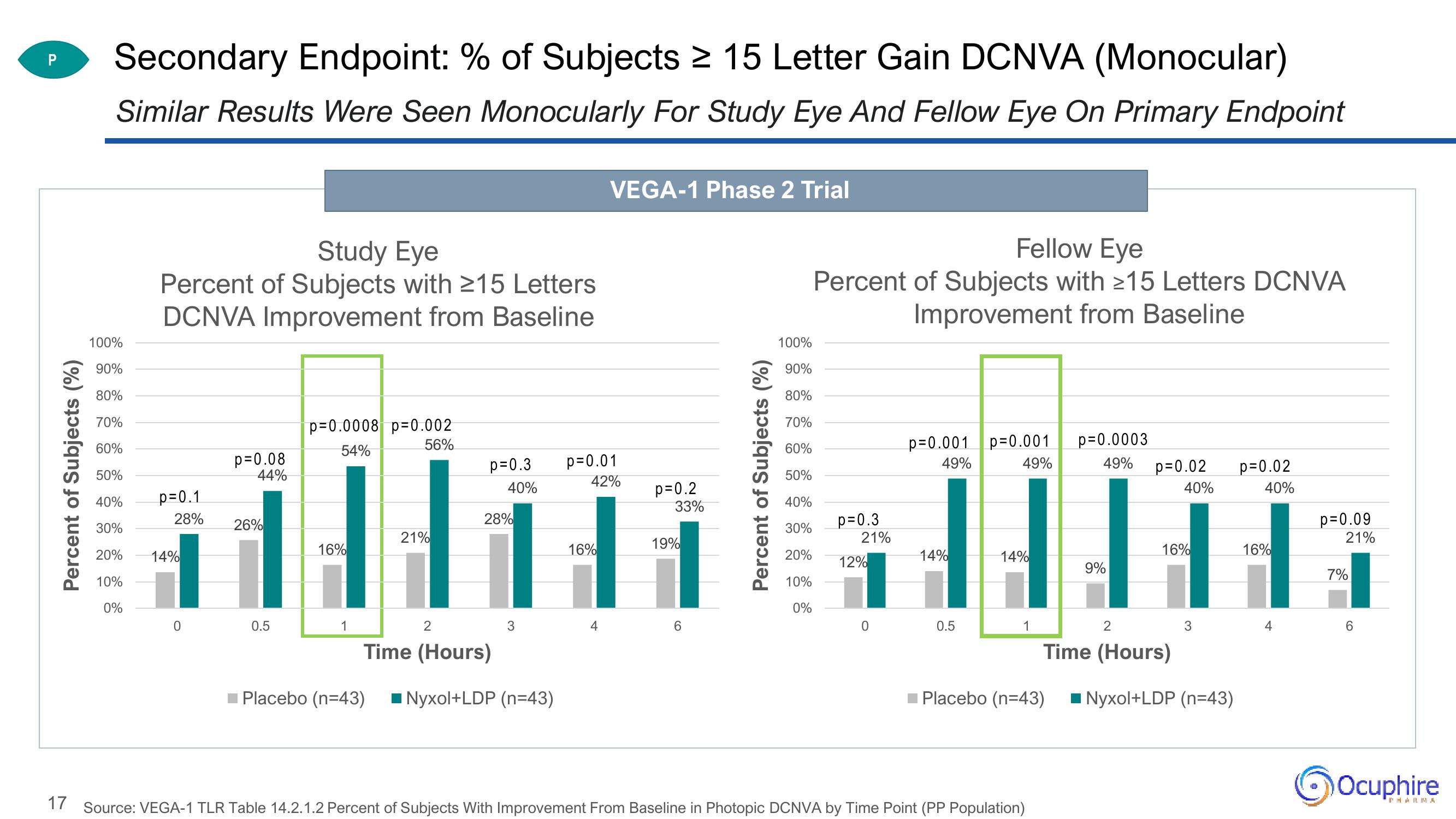
Secondary Endpoint: % of Subjects ≥ 15 Letter Gain DCNVA (Monocular)
Similar Results Were Seen Monocularly For Study Eye And Fellow Eye On Primary Endpoint
Study Eye
Percent of Subjects with ≥15 Letters
DCNVA Improvement from Baseline
100%
90%
80%
70%
60%
50%
40% p=0.1
28%
30%
20%
10%
0%
14%
0
p=0.08
44%
26%
0.5
p=0.0008 p=0.002
54%
56%
16%
1
21%
Placebo (n=43)
2
p=0.3 p=0.01
42%
40%
28%
Time (Hours)
3
Nyxol+LDP (n=43)
16%
VEGA-1 Phase 2 Trial
4
p=0.2
33%
19%
6
Percent of Subjects (%)
100%
90%
80%
70%
60%
50%
40%
30%
20%
10%
0%
Fellow Eye
Percent of Subjects with ≥15 Letters DCNVA
Improvement from Baseline
p=0.3
21%
12%
0
p=0.001 p=0.001
49%
49%
14%
0.5
14%
1
Placebo (n=43)
17 Source: VEGA-1 TLR Table 14.2.1.2 Percent of Subjects With Improvement From Baseline in Photopic DCNVA by Time Point (PP Population)
p=0.0003
49% p=0.02 p=0.02
40%
40%
9%
2
16%
Time (Hours)
3
Nyxol+LDP (n=43)
16%
4
p=0.09
21%
7%
6
Ocuphire
PHARMAView entire presentation