AstraZeneca Results Presentation Deck
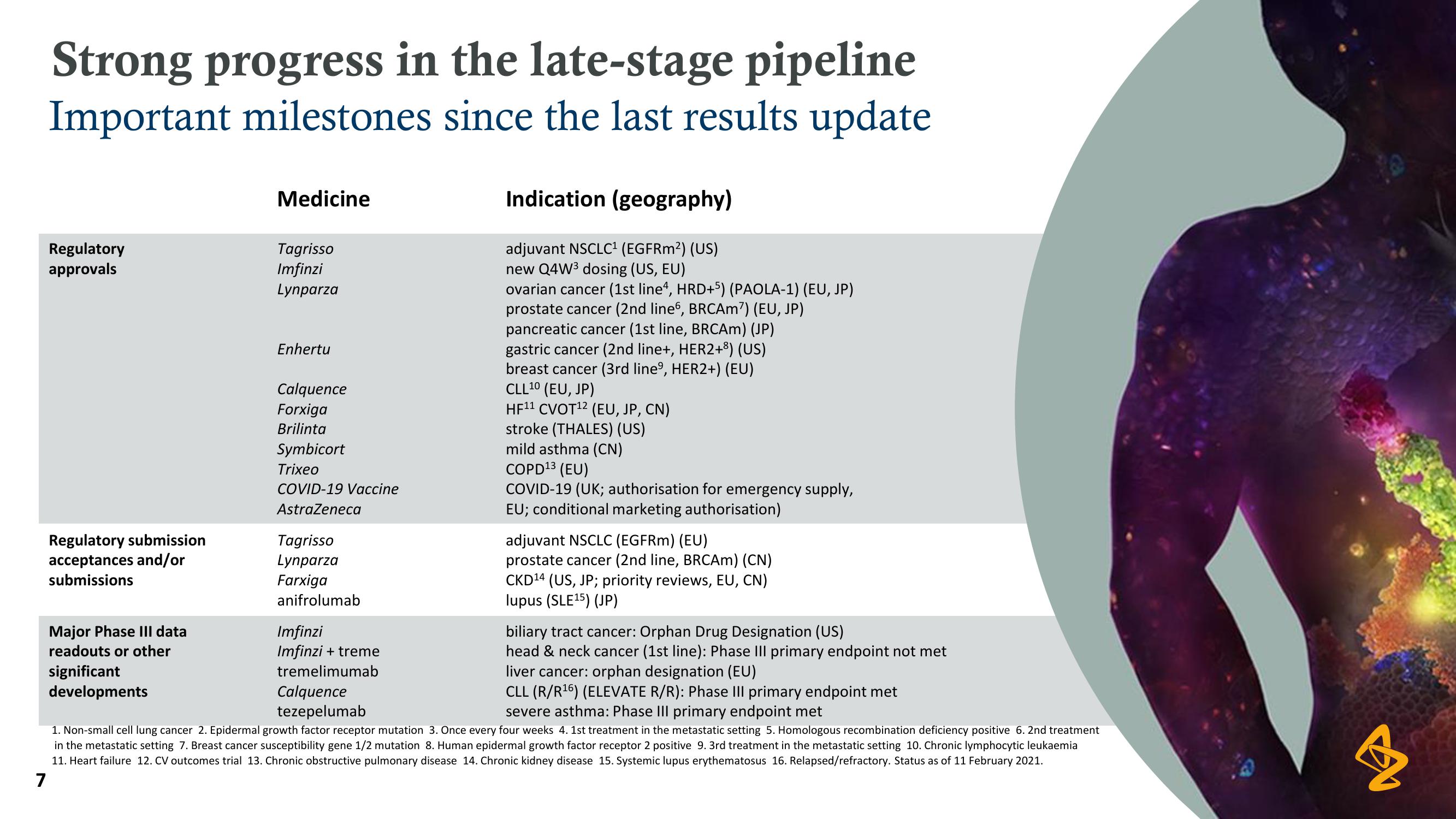
Strong progress in the late-stage pipeline
Important milestones since the last results update
Regulatory
approvals
Regulatory submission
acceptances and/or
submissions
Major Phase III data
readouts or other
significant
developments
Medicine
Tagrisso
Imfinzi
Lynparza
Enhertu
Calquence
Forxiga
Brilinta
Symbicort
Trixeo
COVID-19 Vaccine
AstraZeneca
Tagrisso
Lynparza
Farxiga
anifrolumab
Indication (geography)
adjuvant NSCLC¹ (EGFRm²) (US)
new Q4W³ dosing (US, EU)
ovarian cancer (1st line4, HRD+5) (PAOLA-1) (EU, JP)
prostate cancer (2nd line6, BRCAM7) (EU, JP)
pancreatic cancer (1st line, BRCAM) (JP)
gastric cancer (2nd line+, HER2+³) (US)
breast cancer (3rd lineº, HER2+) (EU)
CLL ¹0 (EU, JP)
HF¹¹ CVOT¹2 (EU, JP, CN)
stroke (THALES) (US)
mild asthma (CN)
COPD ¹3 (EU)
COVID-19 (UK; authorisation for emergency supply,
EU; conditional marketing authorisation)
adjuvant NSCLC (EGFRM) (EU)
prostate cancer (2nd line, BRCAm) (CN)
CKD¹4 (US, JP; priority reviews, EU, CN)
lupus (SLE¹5) (JP)
Imfinzi
biliary tract cancer: Orphan Drug Designation (US)
Imfinzi + treme
head & neck cancer (1st line): Phase III primary endpoint not met
liver cancer: orphan designation (EU)
tremelimumab
Calquence
tezepelumab
CLL (R/R¹6) (ELEVATE R/R): Phase III primary endpoint met
severe asthma: Phase III primary endpoint met
1. Non-small cell lung cancer 2. Epidermal growth factor receptor mutation 3. Once every four weeks 4. 1st treatment in the metastatic setting 5. Homologous recombination deficiency positive 6. 2nd treatment
in the metastatic setting 7. Breast cancer susceptibility gene 1/2 mutation 8. Human epidermal growth factor receptor 2 positive 9. 3rd treatment in the metastatic setting 10. Chronic lymphocytic leukaemia
11. Heart failure 12. CV outcomes trial 13. Chronic obstructive pulmonary disease 14. Chronic kidney disease 15. Systemic lupus erythematosus 16. Relapsed/refractory. Status as of 11 February 2021.
7
4View entire presentation