AstraZeneca Results Presentation Deck
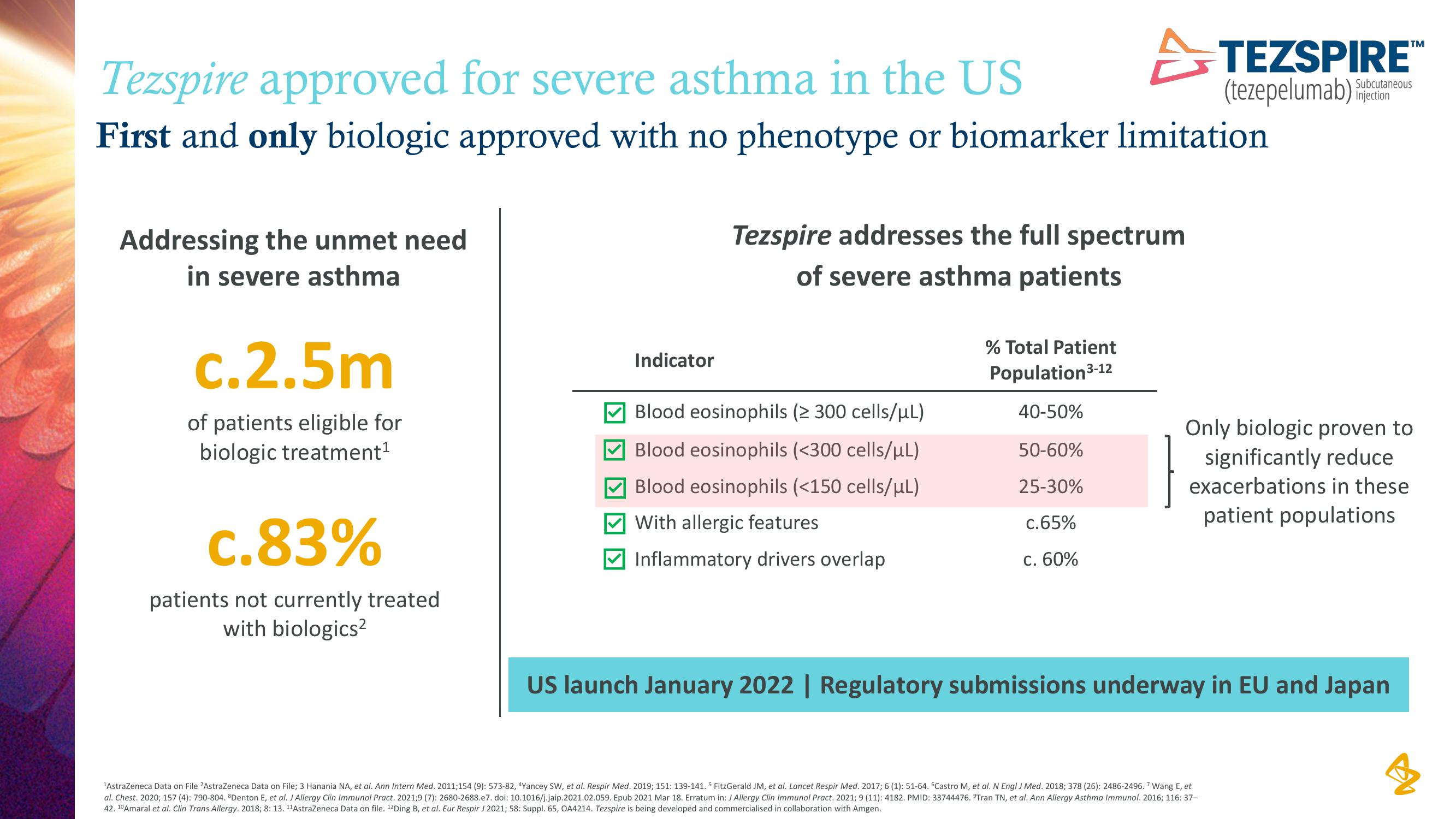
Tezspire approved for severe asthma in the US
First and only biologic approved with no phenotype or biomarker limitation
Addressing the unmet need
in severe asthma
c.2.5m
of patients eligible for
biologic treatment¹
c.83%
patients not currently treated
with biologics²
Indicator
Tezspire addresses the full spectrum
of severe asthma patients
Blood eosinophils (≥ 300 cells/μL)
Blood eosinophils (<300 cells/μL)
Blood eosinophils (<150 cells/μL)
With allergic features
Inflammatory drivers overlap
% Total Patient
Population ³-12
40-50%
50-60%
25-30%
c.65%
c. 60%
TEZSPIRE™
(tezepelumab) Injection
Subcutaneous
Only biologic proven to
significantly reduce
exacerbations in these
patient populations
¹AstraZeneca Data on File ²AstraZeneca Data on File; 3 Hanania NA, et al. Ann Intern Med. 2011;154 (9): 573-82, 4Yancey SW, et al. Respir Med. 2019; 151: 139-141. 5 FitzGerald JM, et al. Lancet Respir Med. 2017; 6 (1): 51-64. "Castro M, et al. N Engl J Med. 2018; 378 (26): 2486-2496.7 Wang E, et
al. Chest. 2020; 157 (4): 790-804. Denton E, et al. J Allergy Clin Immunol Pract. 2021;9 (7): 2680-2688.e7. doi: 10.1016/j.jaip.2021.02.059. Epub 2021 Mar 18. Erratum in: J Allergy Clin Immunol Pract. 2021; 9 (11): 4182. PMID: 33744476. Tran TN, et al. Ann Allergy Asthma Immunol. 2016; 116: 37-
42. 10 Amaral et al. Clin Trans Allergy. 2018; 8: 13. 1¹AstraZeneca Data on file. ¹2Ding B, et al. Eur Respir J 2021; 58: Suppl. 65, OA4214. Tezspire is being developed and commercialised in collaboration with Amgen.
US launch January 2022 | Regulatory submissions underway in EU and JapanView entire presentation