BioNTech Results Presentation Deck
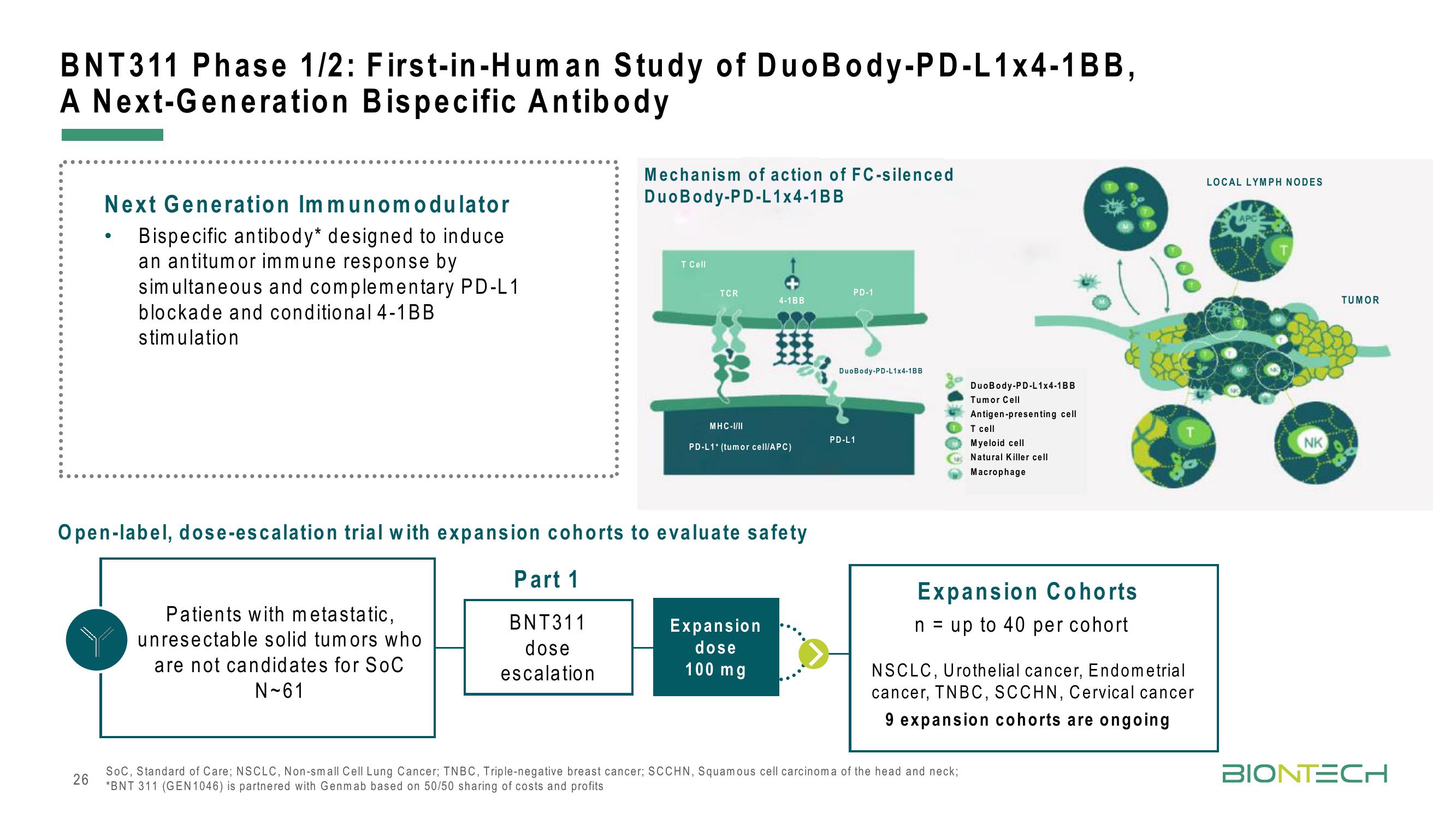
BNT311 Phase 1/2: First-in-Human Study of DuoBody-PD-L1x4-1BB,
A Next-Generation Bispecific Antibody
Next Generation Immunomodulator
Bispecific antibody* designed to induce
an antitumor immune response by
simultaneous and complementary PD-L1
26
blockade and conditional 4-1BB
stimulation
Mechanism of action of FC-silenced
DuoBody-PD-L1x4-1BB
Patients with metastatic,
unresectable solid tumors who
are not candidates for SoC
N~61
T Cell
TCR
MHC-I/II
Open-label, dose-escalation trial with expansion cohorts to evaluate safety
Part 1
BNT311
dose
escalation
4-1BB
PD-L1* (tumor cell/APC)
Expansion
dose
100 mg
PD-1
DuoBody-PD-L1x4-1BB
PD-L1
DuoBody-PD-L1x4-1BB
Tumor Cell
Antigen-presenting cell
T cell
Myeloid cell
Natural Killer cell
Macrophage
Expansion Cohorts
n = up to 40 per cohort
SoC, Standard of Care; NSCLC, Non-small Cell Lung Cancer; TNBC, Triple-negative breast cancer; SCCHN, Squamous cell carcinoma of the head and neck;
*BNT 311 (GEN 1046) is partnered with Genmab based on 50/50 sharing of costs and profits
NSCLC, Urothelial cancer, Endometrial
cancer, TNBC, SCCHN, Cervical cancer
9 expansion cohorts are ongoing
LOCAL LYMPH NODES
APC
NK
TUMOR
BIONTECHView entire presentation