BioAtla Investor Presentation Deck
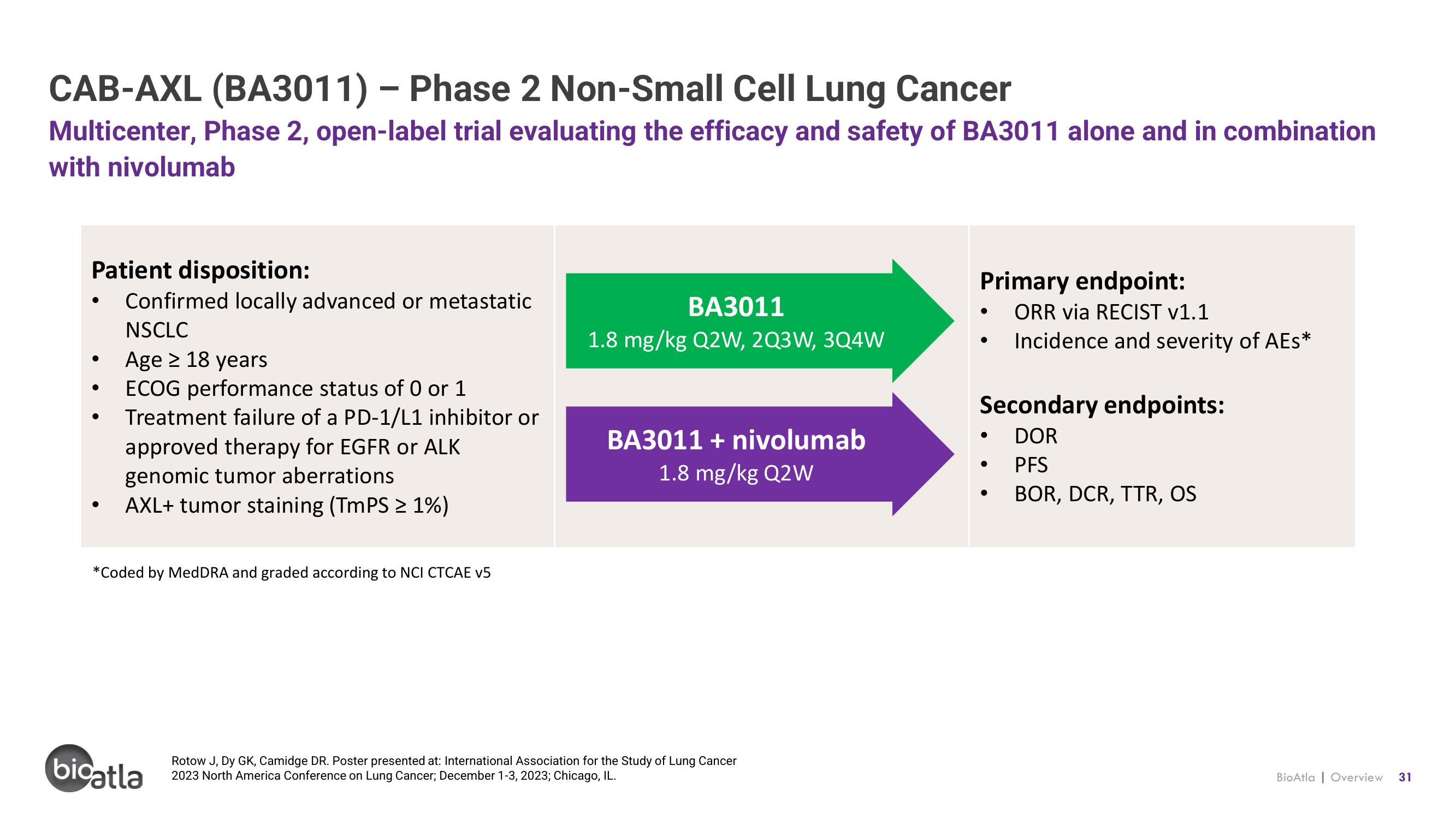
CAB-AXL (BA3011) - Phase 2 Non-Small Cell Lung Cancer
Multicenter, Phase 2, open-label trial evaluating the efficacy and safety of BA3011 alone and in combination
with nivolumab
Patient disposition:
Confirmed locally advanced or metastatic
NSCLC
●
●
●
Age ≥ 18 years
ECOG performance status of 0 or 1
Treatment failure of a PD-1/L1 inhibitor or
approved therapy for EGFR or ALK
genomic tumor aberrations
AXL+ tumor staining (TmPS ≥ 1%)
*Coded by MedDRA and graded according to NCI CTCAE v5
bicatla
BA3011
1.8 mg/kg Q2W, 2Q3W, 3Q4W
BA3011 + nivolumab
1.8 mg/kg Q2W
Rotow J, Dy GK, Camidge DR. Poster presented at: International Association for the Study of Lung Cancer
2023 North America Conference on Lung Cancer; December 1-3, 2023; Chicago, IL.
Primary endpoint:
●
●
ORR via RECIST v1.1
Incidence and severity of AEs*
Secondary endpoints:
DOR
PFS
BOR, DCR, TTR, OS
●
BioAtla| Overview
31View entire presentation