AstraZeneca Investor Day Presentation Deck
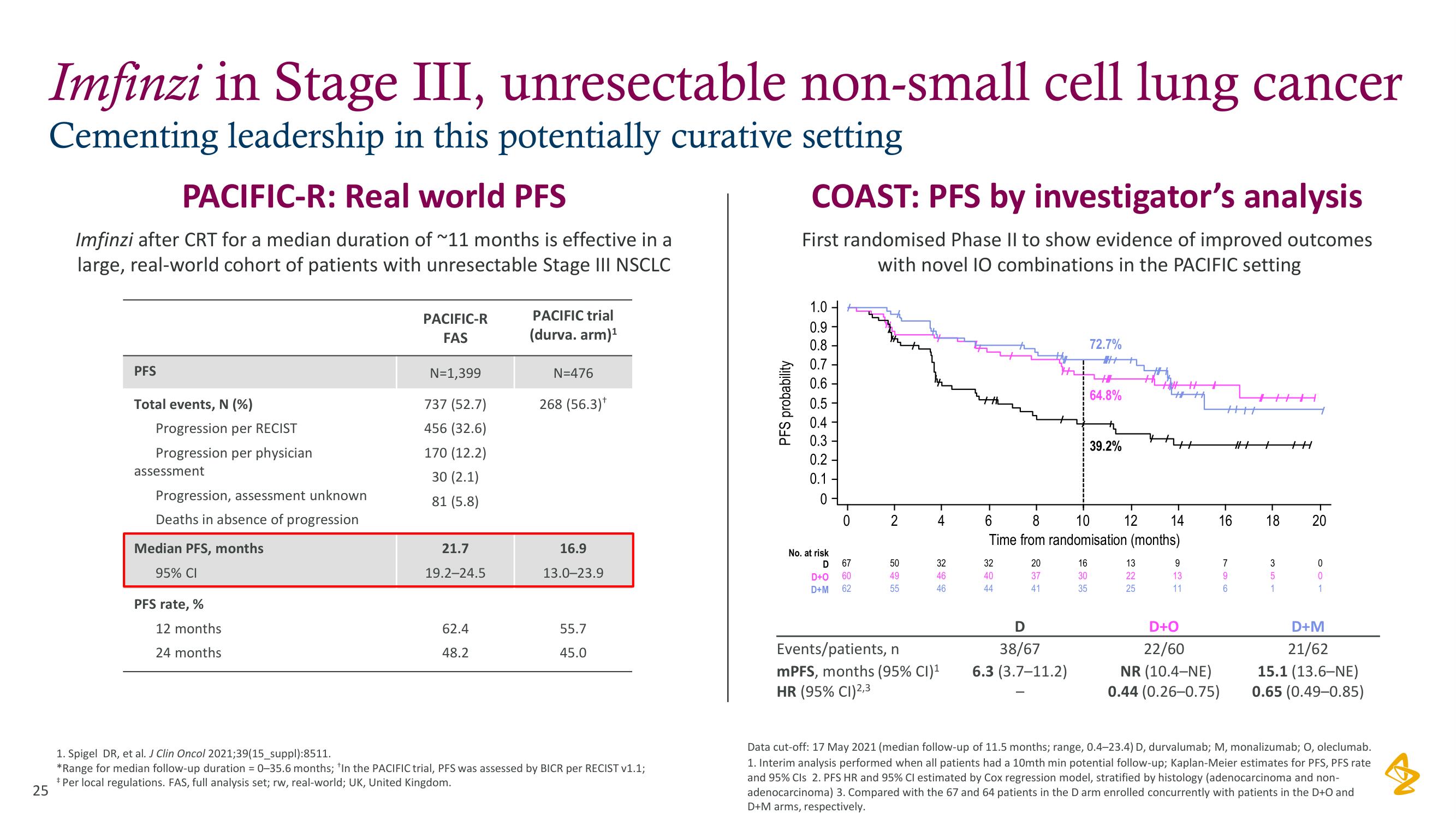
Imfinzi in Stage III, unresectable non-small cell lung cancer
Cementing leadership in this potentially curative setting
PACIFIC-R: Real world PFS
Imfinzi after CRT for a median duration of ~11 months is effective in a
large, real-world cohort of patients with unresectable Stage III NSCLC
PFS
Total events, N (%)
Progression per RECIST
Progression per physician
assessment
Progression, assessment unknown
Deaths in absence of progression
Median PFS, months
95% CI
PFS rate, %
12 months
24 months
PACIFIC-R
FAS
N=1,399
737 (52.7)
456 (32.6)
170 (12.2)
30 (2.1)
81 (5.8)
21.7
19.2-24.5
62.4
48.2
PACIFIC trial
(durva. arm)¹
N=476
268 (56.3)*
16.9
13.0-23.9
55.7
45.0
1. Spigel DR, et al. J Clin Oncol 2021;39(15_suppl):8511.
*Range for median follow-up duration = 0-35.6 months; *In the PACIFIC trial, PFS was assessed by BICR per RECIST v1.1;
* Per local regulations. FAS, full analysis set; rw, real-world; UK, United Kingdom.
25
PFS probability
COAST: PFS by investigator's analysis
First randomised Phase II to show evidence of improved outcomes
with novel 10 combinations in the PACIFIC setting
1.0
0.9
0.8
0.7
765432
0.6
0.5
o o o o
0.4
0.3
0.2
0.1
0
No. at risk
D
O.
0
67
60
D+O
D+M 62
T
2
50
49
55
4
32
46
46
Events/patients, n
mPFS, months (95% CI)¹
HR (95% CI)2,3
32
40
44
20
37
41
D
38/67
6.3 (3.7-11.2)
72.7%
6
8
10
Time from randomisation (months)
16
30
35
64.8%
39.2%
T
12 14
13
22
25
9
13
11
H|||
T
16
D+O
22/60
NR (10.4-NE)
0.44 (0.26-0.75)
7
9
6
T
18
3
5
1
20
0
0
1
D+M
21/62
15.1 (13.6-NE)
0.65 (0.49-0.85)
Data cut-off: 17 May 2021 (median follow-up of 11.5 months; range, 0.4-23.4) D, durvalumab; M, monalizumab; O, oleclumab.
1. Interim analysis performed when all patients had a 10mth min potential follow-up; Kaplan-Meier estimates for PFS, PFS rate
and 95% Cls 2. PFS HR and 95% CI estimated by Cox regression model, stratified by histology (adenocarcinoma and non-
adenocarcinoma) 3. Compared with the 67 and 64 patients in the D arm enrolled concurrently with patients in the D+O and
D+M arms, respectively.View entire presentation