AstraZeneca Investor Day Presentation Deck
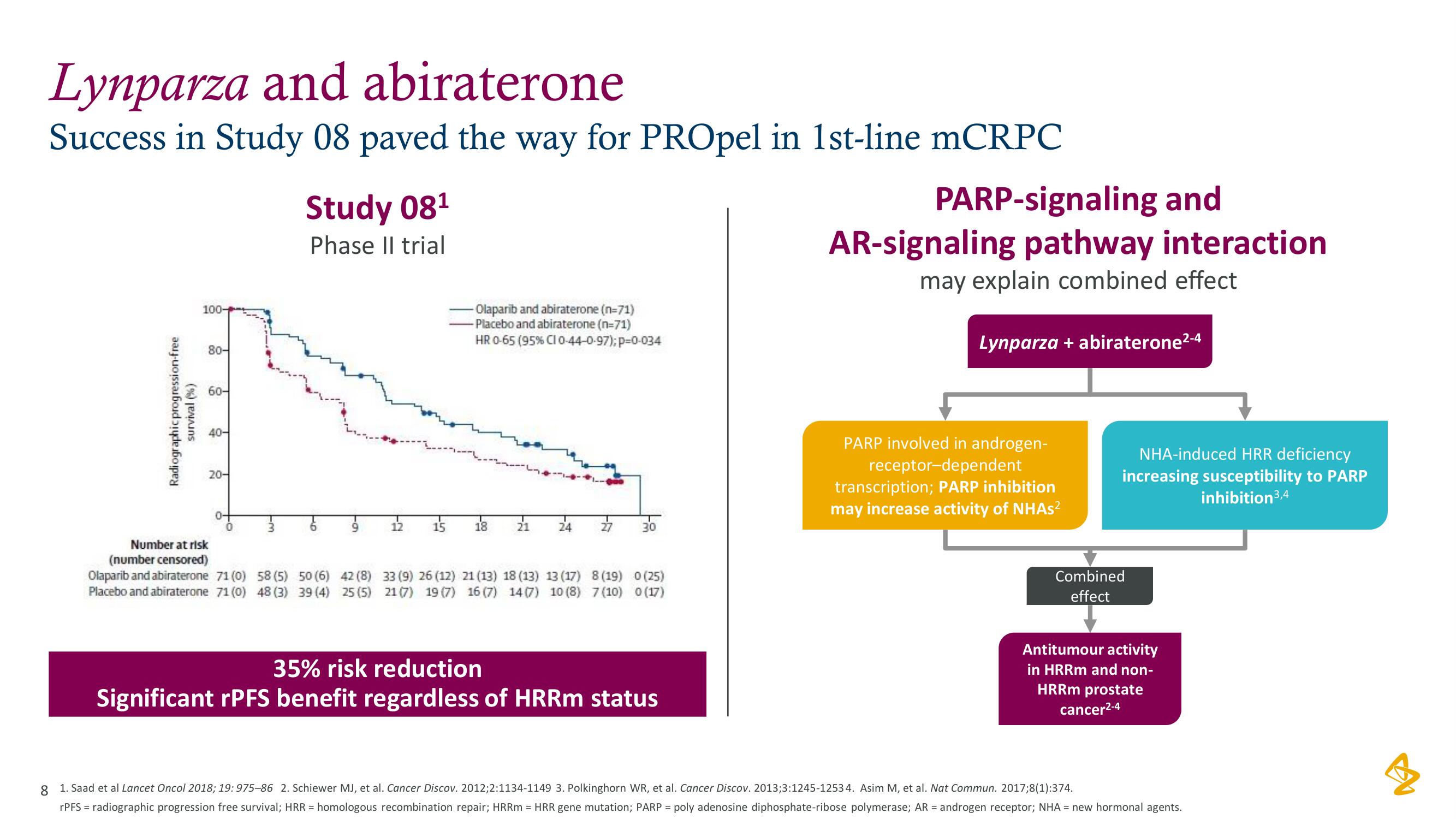
Lynparza and abiraterone
Success in Study 08 paved the way for PROpel in 1st-line mCRPC
Radiographic progression-free
survival (%)
100-
80-
60-
40-
20-
Number at risk
(number censored)
Olaparib and abiraterone 71 (0)
Placebo and abiraterone 71 (0)
Study 08¹
Phase II trial
T6
9
58 (5) 50 (6) 42 (8)
48 (3) 39 (4) 25 (5)
12
15
- Olaparib and abiraterone (n=71)
-Placebo and abiraterone (n=71)
HR 0-65 (95% CI 0-44-0-97); p=0-034
18
21
24
33 (9) 26 (12) 21 (13) 18 (13) 13 (17) 8 (19) 0 (25)
21 (7) 19 (7) 16 (7) 14 (7) 10 (8) 7 (10) 0 (17)
35% risk reduction
Significant rPFS benefit regardless of HRRm status
PARP-signaling and
AR-signaling pathway interaction
may explain combined effect
Lynparza + abiraterone²-4
PARP involved in androgen-
receptor-dependent
transcription; PARP inhibition
may increase activity of NHAs²
NHA-induced HRR deficiency
increasing susceptibility to PARP
inhibition 3,4
Combined
effect
Antitumour activity
in HRRm and non-
HRRm prostate
cancer²-4
8 1. Saad et al Lancet Oncol 2018; 19: 975-86 2. Schiewer MJ, et al. Cancer Discov. 2012;2:1134-1149 3. Polkinghorn WR, et al. Cancer Discov. 2013;3:1245-12534. Asim M, et al. Nat Commun. 2017;8(1):374.
rPFS = radiographic progression free survival; HRR = homologous recombination repair; HRRm = HRR gene mutation; PARP = poly adenosine diphosphate-ribose polymerase; AR = androgen receptor; NHA = new hormonal agents.View entire presentation