Ocuphire Pharma Results
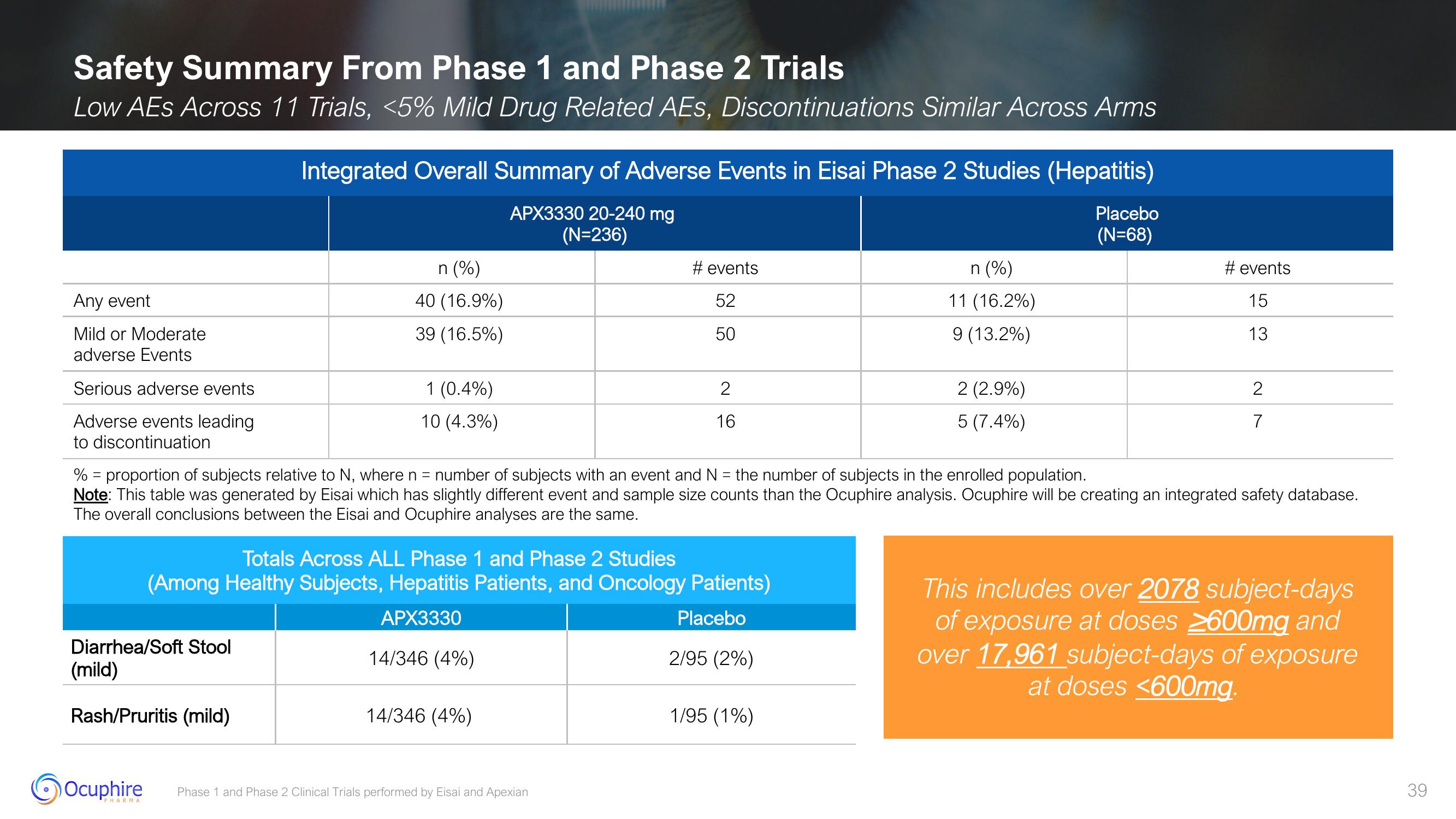
Safety Summary From Phase 1 and Phase 2 Trials
Low AES Across 11 Trials, <5% Mild Drug Related AES, Discontinuations Similar Across Arms
Any event
Mild or Moderate
adverse Events
Serious adverse events
Adverse events leading
to discontinuation
Diarrhea/Soft Stool
(mild)
Integrated Overall Summary of Adverse Events in Eisai Phase 2 Studies (Hepatitis)
APX3330 20-240 mg
(N=236)
Rash/Pruritis (mild)
n (%)
40 (16.9%)
39 (16.5%)
PHARMA
1 (0.4%)
10 (4.3%)
Totals Across ALL Phase 1 and Phase 2 Studies
(Among Healthy Subjects, Hepatitis Patients, and Oncology Patients)
APX3330
Placebo
14/346 (4%)
# events
52
50
14/346 (4%)
Ocuphire Phase 1 and Phase 2 Clinical Trials performed by Eisai and Apexian
2
16
% = proportion of subjects relative to N, where n = number of subjects with an event and N = the number of subjects in the enrolled population.
Note: This table was generated by Eisai which has slightly different event and sample size counts than the Ocuphire analysis. Ocuphire will be creating an integrated safety database.
The overall conclusions between the Eisai and Ocuphire analyses are the same.
2/95 (2%)
n (%)
11 (16.2%)
9 (13.2%)
1/95 (1%)
2 (2.9%)
5 (7.4%)
Placebo
(N=68)
#events
15
13
2
7
This includes over 2078 subject-days
of exposure at doses 2600mg and
over 17,961 subject-days of exposure
at doses <600mg.
39View entire presentation