Ocuphire Pharma Investor Day Presentation Deck
RM
49
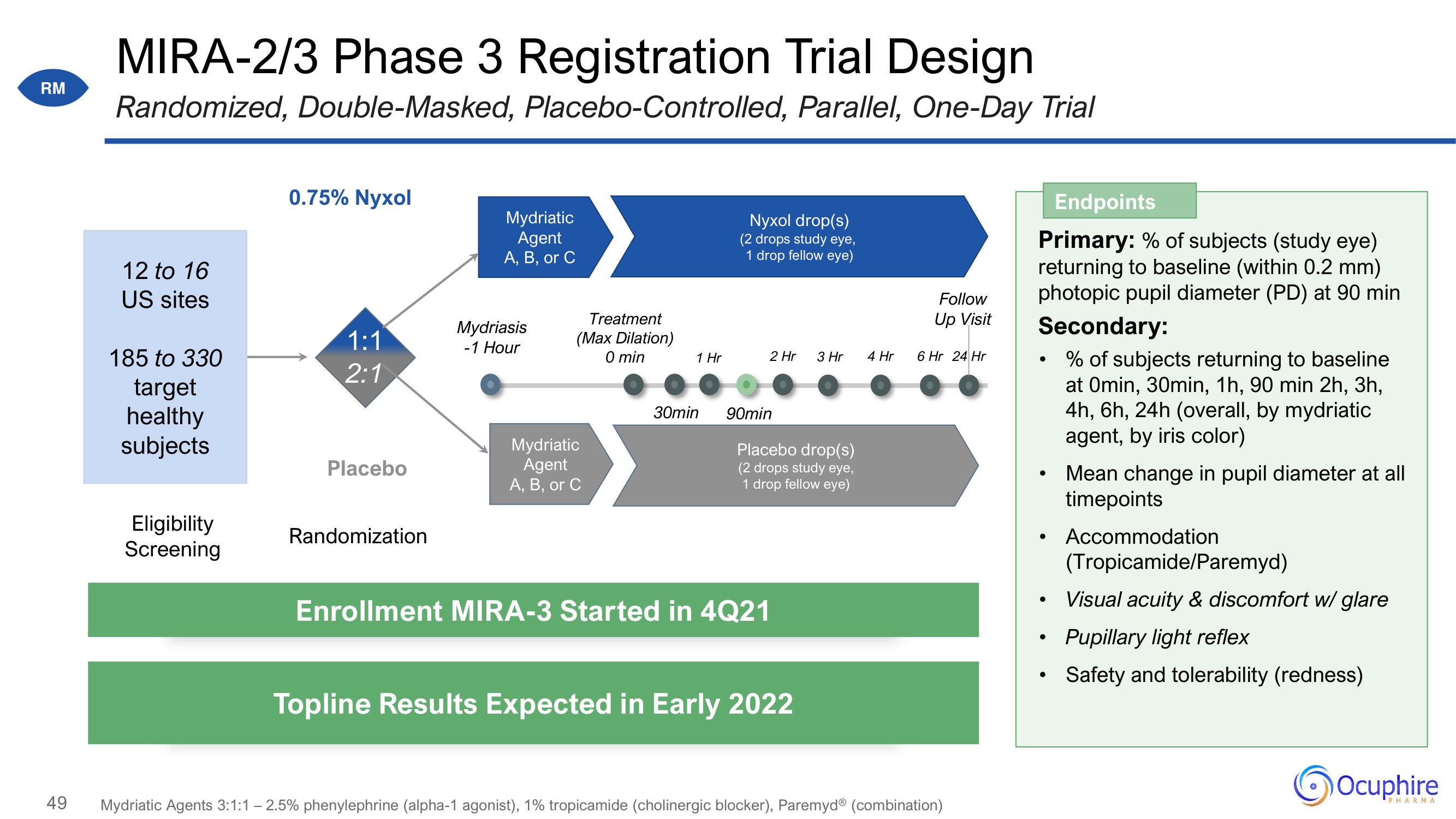
MIRA-2/3 Phase 3 Registration Trial Design
Randomized, Double-Masked, Placebo-Controlled, Parallel, One-Day Trial
12 to 16
US sites
185 to 330
target
healthy
subjects
Eligibility
Screening
0.75% Nyxol
1:1
2:1
Placebo
Randomization
Mydriatic
Agent
A, B, or C
Mydriasis
-1 Hour
Treatment
(Max Dilation)
0 min
Mydriatic
Agent
A, B, or C
1 Hr
30min
Nyxol drop(s)
(2 drops study eye,
1 drop fellow eye)
2 Hr 3 Hr
90min
Placebo drop(s)
(2 drops study eye,
1 drop fellow eye)
Enrollment MIRA-3 Started in 4Q21
Topline Results Expected in Early 2022
4 Hr
Follow
Up Visit
6 Hr 24 Hr
Mydriatic Agents 3:1:1 - 2.5% phenylephrine (alpha-1 agonist), 1% tropicamide (cholinergic blocker), Paremyd® (combination)
Endpoints
Primary: % of subjects (study eye)
returning to baseline (within 0.2 mm)
photopic pupil diameter (PD) at 90 min
Secondary:
●
●
●
●
●
% of subjects returning to baseline
at Omin, 30min, 1h, 90 min 2h, 3h,
4h, 6h, 24h (overall, by mydriatic
agent, by iris color)
Mean change in pupil diameter at all
timepoints
Accommodation
(Tropicamide/Paremyd)
Visual acuity & discomfort w/ glare
Pupillary light reflex
Safety and tolerability (redness)
Ocuphire
PHARMAView entire presentation