Baran Group Meeting
Terry Lou
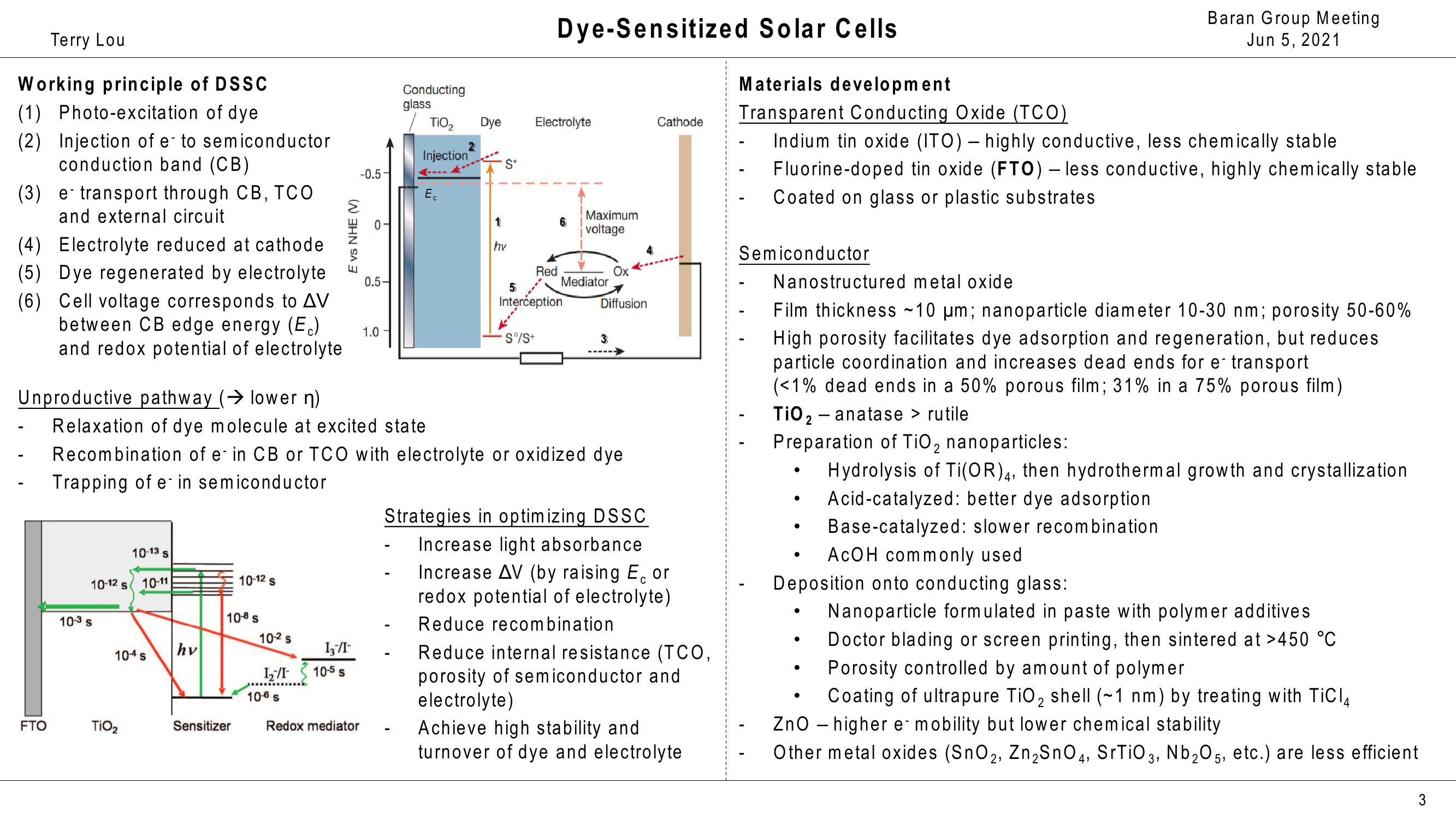
Working principle of DSSC
(1) Photo-excitation of dye
(2) Injection of e- to semiconductor
conduction band (CB)
(3) e-transport through CB, TCO
and external circuit
(4) Electrolyte reduced at cathode
(5) Dye regenerated by electrolyte
(6) Cell voltage corresponds to AV
between CB edge energy (Ec)
and redox potential of electrolyte
Unproductive pathway (→ lower n)
-
E vs NHE (V
-0.5-
Conducting
glass
TiO2
Injection
Ec
Dye-Sensitized Solar Cells
Dye
Electrolyte
Cathode
2
S*
1
6
Maximum
voltage
hv
Red
Ox
Mediator
5
Interception
Diffusion
1.0
S%/S+
3
0.5-
Relaxation of dye molecule at excited state
Recombination of e- in CB or TCO with electrolyte or oxidized dye
-
Trapping of e in semiconductor
10-3 s
10-13 s
10-12 s 10-11
10-12 s
10-8 s
10-2 s
hv
104 s
12/I-
10-6 s
13/I-
10-5 s
FTO
TiO2
Sensitizer
Redox mediator
Strategies in optimizing DSSC
-
C
Increase light absorbance
Increase AV (by raising Ę or
redox potential of electrolyte)
Reduce recombination
Reduce internal resistance (TCO,
porosity of semiconductor and
electrolyte)
Achieve high stability and
turnover of dye and electrolyte
Materials development
Transparent Conducting Oxide (TCO)
Baran Group Meeting
Jun 5, 2021
Indium tin oxide (ITO) – highly conductive, less chemically stable
Fluorine-doped tin oxide (FTO) - less conductive, highly chemically stable
Coated on glass or plastic substrates
Semiconductor
Nanostructured metal oxide
Film thickness ~10 μm; nanoparticle diameter 10-30 nm; porosity 50-60%
High porosity facilitates dye adsorption and regeneration, but reduces
particle coordination and increases dead ends for e- transport
(<1% dead ends in a 50% porous film; 31% in a 75% porous film)
TiO2 - anatase > rutile
Preparation of TiO 2 nanoparticles:
.
•
•
•
Hydrolysis of Ti(OR) 4, then hydrothermal growth and crystallization
Acid-catalyzed: better dye adsorption
Base-catalyzed: slower recombination
AcOH commonly used
Deposition onto conducting glass:
•
Nanoparticle formulated in paste with polymer additives
•
Doctor blading or screen printing, then sintered at >450 °C
•
Porosity controlled by amount of polymer
Coating of ultrapure TiO 2 shell (~1 nm) by treating with TiCl4
ZnO higher e mobility but lower chemical stability
Other metal oxides (SnO 2, Zn2SnO 4, SrTiO 3, Nb2O5, etc.) are less efficient
3View entire presentation