Matinas BioPharma Investor Presentation Deck
16
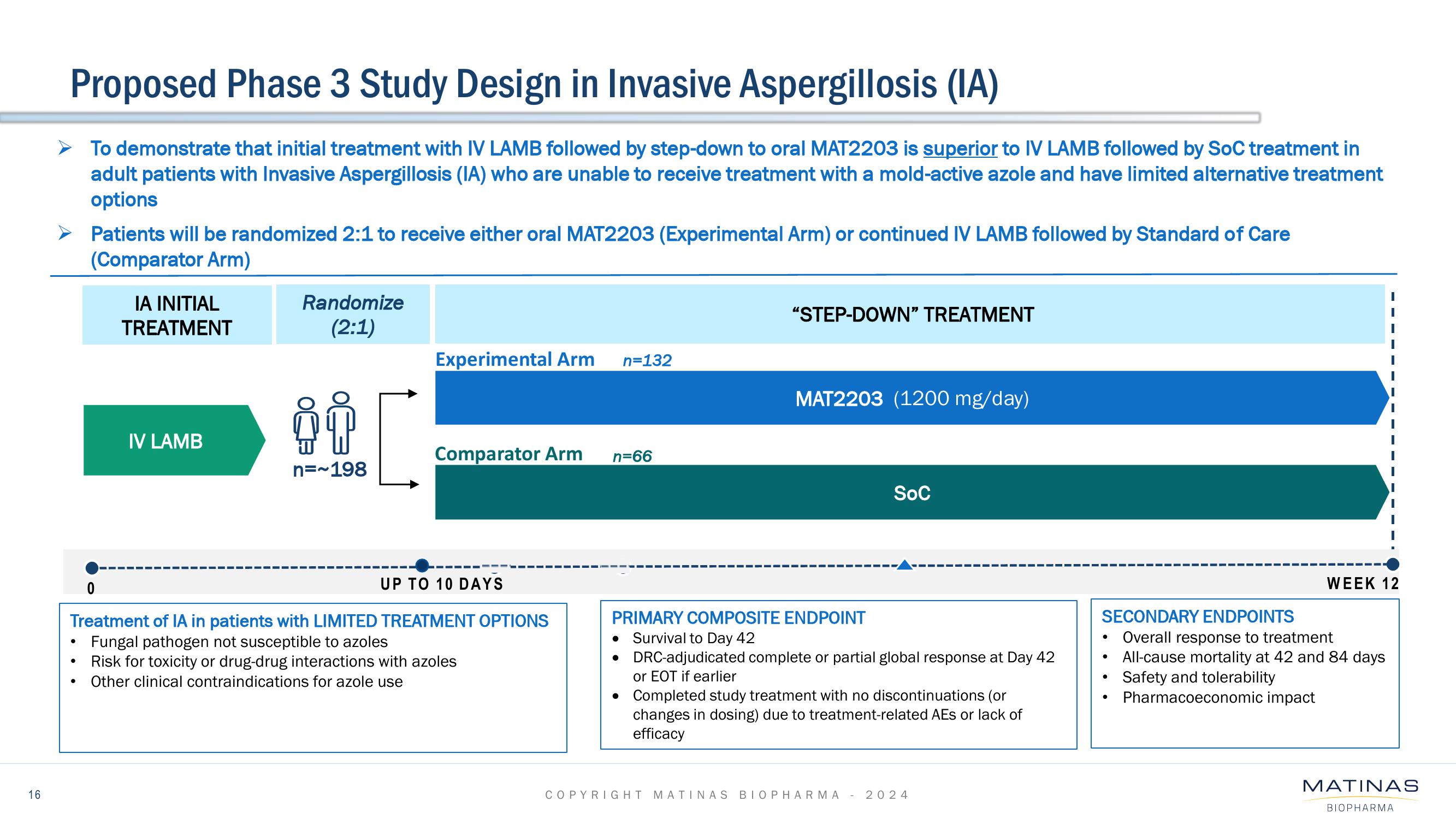
Proposed Phase 3 Study Design in Invasive Aspergillosis (IA)
To demonstrate that initial treatment with IV LAMB followed by step-down to oral MAT2203 is superior to IV LAMB followed by SoC treatment in
adult patients with Invasive Aspergillosis (IA) who are unable to receive treatment with a mold-active azole and have limited alternative treatment
options
●
Patients will be randomized 2:1 to receive either oral MAT2203 (Experimental Arm) or continued IV LAMB followed by Standard of Care
(Comparator Arm)
●
IA INITIAL
TREATMENT
IV LAMB
Randomize
(2:1)
oi
n=~198
Experimental Arm
Comparator Arm
Treatment of IA in patients with LIMITED TREATMENT OPTIONS
Fungal pathogen not susceptible to azoles
Risk for toxicity or drug-drug interactions with azoles
Other clinical contraindications for azole use
UP TO 10 DAYS
n=132
n=66
●
"STEP-DOWN" TREATMENT
MAT2203 (1200 mg/day)
PRIMARY COMPOSITE ENDPOINT
Survival to Day 42
• DRC-adjudicated complete or partial global response at Day 42
or EOT if earlier
•
SOC
Completed study treatment with no discontinuations (or
changes in dosing) due to treatment-related AES or lack of
efficacy
COPYRIGHT MATINAS BIOPHARMA 2024
-
WEEK 12
SECONDARY ENDPOINTS
Overall response to treatment
All-cause mortality at 42 and 84 days
Safety and tolerability
Pharmacoeconomic impact
●
MATINAS
BIOPHARMAView entire presentation