Bausch+Lomb Results Presentation Deck
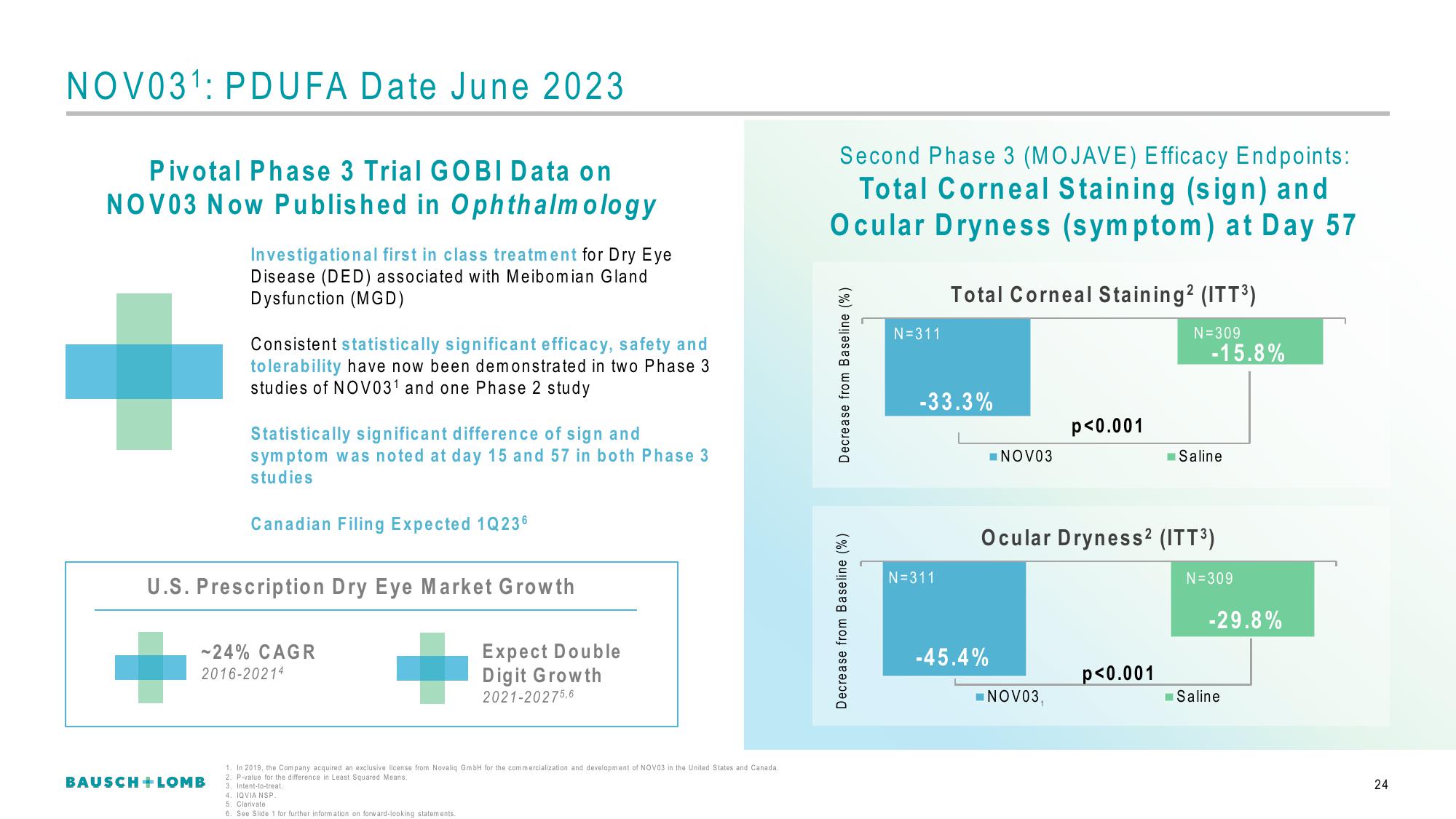
NOV031: PDUFA Date June 2023
Pivotal Phase 3 Trial GOBI Data on
NOV03 Now Published in Ophthalmology
Investigational first in class treatment for Dry Eye
Disease (DED) associated with Meibomian Gland
Dysfunction (MGD)
Consistent statistically significant efficacy, safety and
tolerability have now been demonstrated in two Phase 3
studies of NOV03¹ and one Phase 2 study
BAUSCH + LOMB
Statistically significant difference of sign and
symptom was noted at day 15 and 57 in both Phase 3
studies
Canadian Filing Expected 1Q236
U.S. Prescription Dry Eye Market Growth
-24% CAGR
2016-20214
Expect Double
Digit Growth
2021-20275,6
1. In 2019, the Company acquired an exclusive license from Novaliq GmbH for the commercialization and development of NOV03 in the United States and Canada.
2. P-value for the difference in Least Squared Means.
3. Intent-to-treat.
4. IQVIA NSP.
5. Clarivate
6. See Slide 1 for further information on forward-looking statements.
Second Phase 3 (MOJAVE) Efficacy Endpoints:
Total Corneal Staining (sign) and
Ocular Dryness (symptom) at Day 57
Decrease from Baseline (%)
Decrease from Baseline (%)
N=311
Total Corneal Staining² (ITT³)
N=309
-33.3%
N=311
■NOV03
-45.4%
p<0.001
NOV03,
Ocular Dryness² (ITT³)
-15.8%
p<0.001
Saline
N=309
-29.8%
Saline
24View entire presentation