Ocuphire Pharma Investor Presentation Deck
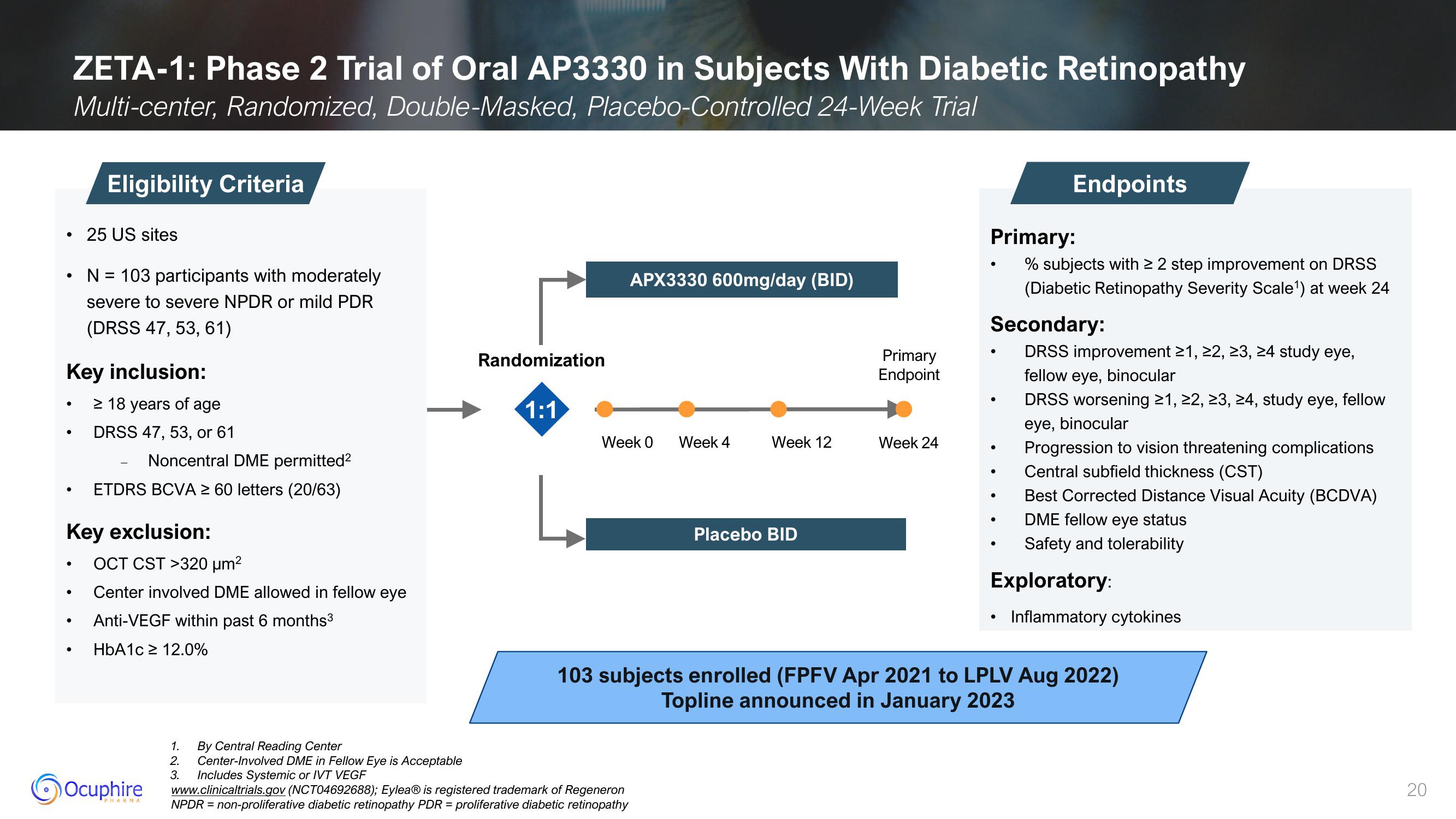
• 25 US sites
●
ZETA-1: Phase 2 Trial of Oral AP3330 in Subjects With Diabetic Retinopathy
Multi-center, Randomized, Double-Masked, Placebo-Controlled 24-Week Trial
●
●
Key inclusion:
●
Eligibility Criteria
.
N = 103 participants with moderately
severe to severe NPDR or mild PDR
(DRSS 47, 53, 61)
●
Key exclusion:
≥ 18 years of age
DRSS 47, 53, or 61
Noncentral DME permitted²
ETDRS BCVA ≥ 60 letters (20/63)
OCT CST >320 μm²
Center involved DME allowed in fellow eye
Anti-VEGF within past 6 months³
HbA1c ≥ 12.0%
Ocuphire
PHARMA
By Central Reading Center
Center-Involved DME in Fellow Eye is Acceptable
1.
2.
3. Includes Systemic or IVT VEGF
Randomization
1:1
APX3330 600mg/day (BID)
Week 0
www.clinicaltrials.gov (NCT04692688); Eylea® is registered trademark of Regeneron
NPDR = non-proliferative diabetic retinopathy PDR = proliferative diabetic retinopathy
Week 4
Week 12
Placebo BID
Primary
Endpoint
Week 24
Primary:
% subjects with ≥ 2 step improvement on DRSS
(Diabetic Retinopathy Severity Scale¹) at week 24
Secondary:
DRSS improvement ≥1, ≥2, ≥3, ≥4 study eye,
fellow eye, binocular
DRSS worsening ≥1, ≥2, ≥3, ≥4, study eye, fellow
eye, binocular
●
●
●
●
Endpoints
●
Progression to vision threatening complications
Central subfield thickness (CST)
Best Corrected Distance Visual Acuity (BCDVA)
DME fellow eye status
Safety and tolerability
Exploratory:
Inflammatory cytokines
103 subjects enrolled (FPFV Apr 2021 to LPLV Aug 2022)
Topline announced in January 2023
20View entire presentation