AstraZeneca Results Presentation Deck
Building new standard of care: Tagrisso
Moving into earlier lines of NSCLC, reshaping the standard of care
Probability of
progression-free survival
1.0-
0.8-
0.6-
0.4-
0.2-
0-
0
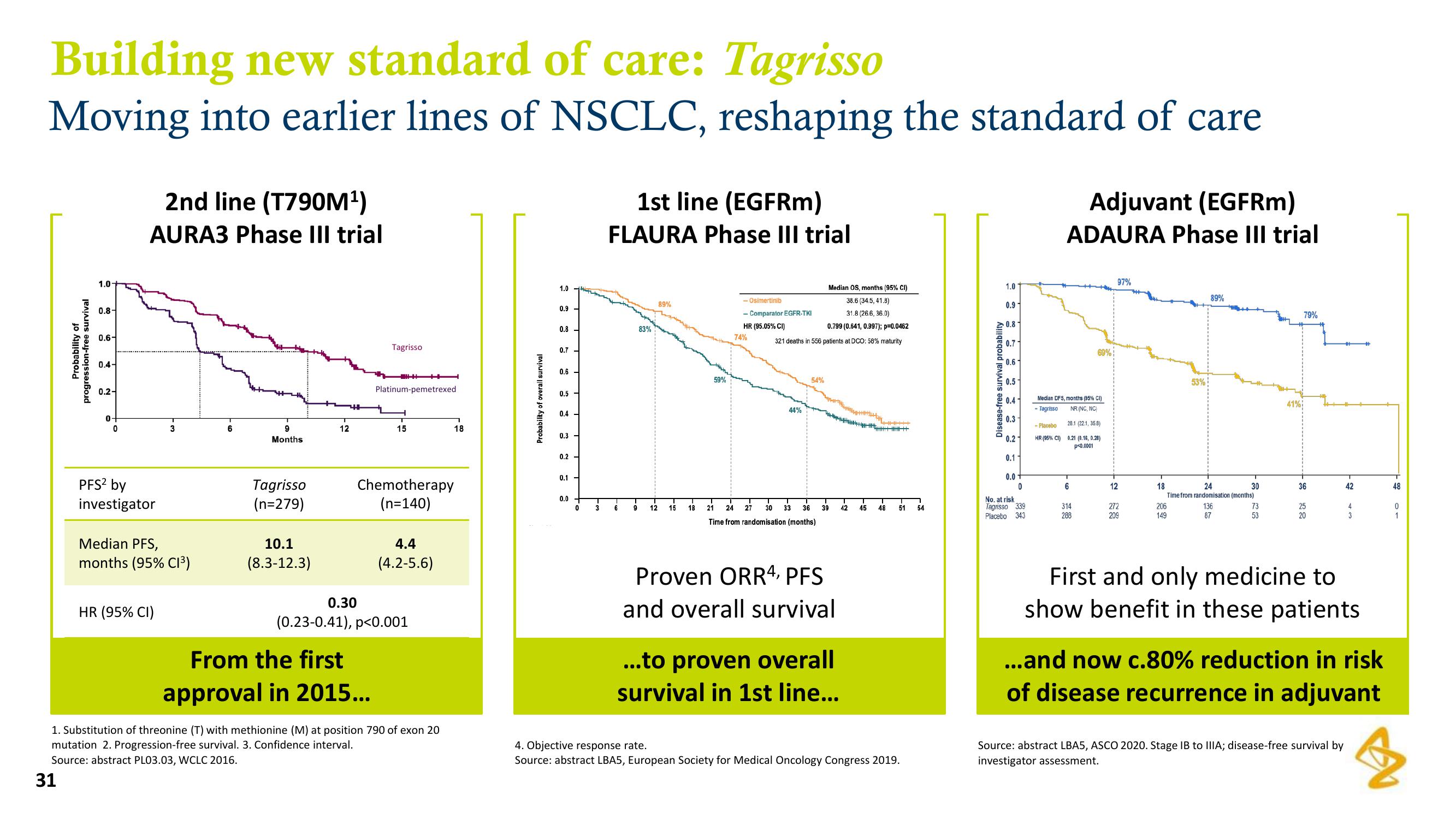
2nd line (T790M¹)
AURA3 Phase III trial
PFS² by
investigator
3
Median PFS,
months (95% C1³)
HR (95% CI)
6
9
Months
Tagrisso
(n=279)
10.1
(8.3-12.3)
12
0.30
Tagrisso
Platinum-pemetrexed
From the first
approval in 2015...
15
Chemotherapy
(n=140)
4.4
(4.2-5.6)
(0.23-0.41), p<0.001
18
1. Substitution of threonine (T) with methionine (M) at position 790 of exon 20
mutation 2. Progression-free survival. 3. Confidence interval.
Source: abstract PL03.03, WCLC 2016.
31
Probability of overall survival
1.0
0.9-
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1-
0.0
0
T
3
1st line (EGFRm)
FLAURA Phase III trial
6
9
83%
89%
12 15
18
59%
-Osimertinib
- Comparator EGFR-TKI
HR (95.05% CI)
74%
321 deaths in 556 patients at DCO: 58% maturity
44%
54%
Median OS, months (95% CI)
38.6 (34.5, 41.8)
31.8 (26.6, 36.0)
0.799 (0.641, 0.997); p=0.0462
21 24 27 30 33 36
Time from randomisation (months)
T
39
Proven ORR4, PFS
and overall survival
...to proven overall
survival in 1st line...
42
T
45
++++
48 51
4. Objective response rate.
Source: abstract LBA5, European Society for Medical Oncology Congress 2019.
54
Disease-free survival probability
1.0
0.9
0.8
0.7
0.6-
0.5-
0.4
0.3
0.2
0.1
0.0
0
No. at risk
Tagrisso 339
Placebo 343
Adjuvant (EGFRm)
ADAURA Phase III trial
- Placebo
HR (95% CI)
Median DFS, months (95% CI)
Tagrisso
NR (NC, NC)
28.1 (22.1, 35.8)
0.21 (0.16, 0.28)
p<0.0001
T
6
60%
314
288
97%
12
272
209
T
18
53%
206
149
89%
24
30
Time from randomisation (months)
136
356
87
73
53
41%
79%
36
25
20
42
4
3
First and only medicine to
show benefit in these patients
Source: abstract LBA5, ASCO 2020. Stage IB to IIIA; disease-free survival by
investigator assessment.
...and now c.80% reduction in risk
of disease recurrence in adjuvant
48
0
1
3View entire presentation