Kymera Investor Presentation Deck
KT-253: Phase 1, Multicenter, Dose-Escalation and Expansion Trial to
Evaluate KT-333 in Adult Patients with Liquid and Solid Tumors
Hematological
Malignancies
●
AML identified as initial
indication based on strong
pre-clinical KT-253 activity
Developed patient
stratification strategy to
target subsets of leukemias
most sensitive to KT-253 as
mono- and combination
therapy
Preclinical data also support
potential development in
other heme indications, such
as ALL and P53WT
lymphomas
Solid Tumors
Preclinical studies have
identified several solid tumor
types sensitive to KT-253
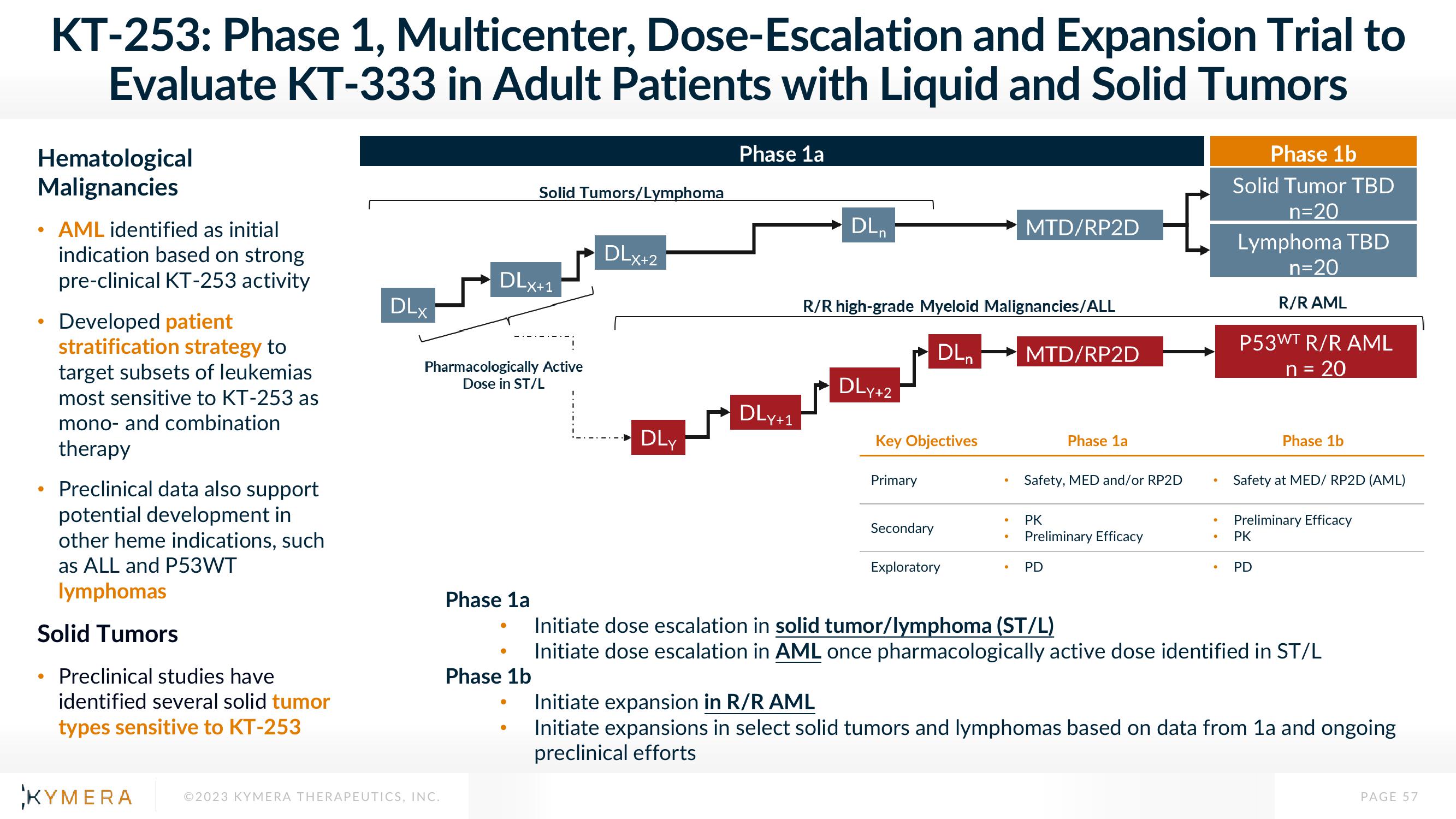
DLX
KYMERA ©2023 KYMERA THERAPEUTICS, INC.
DLx+1
Pharmacologically Active
Dose in ST/L
Solid Tumors/Lymphoma
Phase 1a
Phase 1b
DLx+2
DLy
Phase 1a
DLY+1
DLn
R/R high-grade Myeloid Malignancies/ALL
DL₁
DLY +2
Key Objectives
Primary
Secondary
Exploratory
MTD/RP2D
●
MTD/RP2D
Phase 1a
Safety, MED and/or RP2D
PK
Preliminary Efficacy
PD
.
Phase 1b
Solid Tumor TBD
n=20
Lymphoma TBD
n=20
R/RAML
P53WT R/R AML
n = 20
Phase 1b
Safety at MED/ RP2D (AML)
Preliminary Efficacy
PK
PD
Initiate dose escalation in solid tumor/lymphoma (ST/L)
Initiate dose escalation in AML once pharmacologically active dose identified in ST/L
Initiate expansion in R/R AML
Initiate expansions in select solid tumors and lymphomas based on data from 1a and ongoing
preclinical efforts
PAGE 57View entire presentation